Cutaneous lymphangitis carcinomatosa of breast cancer: a series of 7 cases
Fatima-Zahra Kettani 1, Fouzia Hali1, Kenza Baline1, Farida Marnissi2, Soumia Chiheb1
1, Fouzia Hali1, Kenza Baline1, Farida Marnissi2, Soumia Chiheb1
1Department of Dermatology’s Diseases, CHU Ibn Rochd Casablanca, Morocco; 2Department of Anatomopathology, CHU Ibn Rochd Casablanca, Morocco
Corresponding author: Dr. Fatima-Zahra Kettani
Submission: 13.01.2020; Acceptance: 19.03.2020
DOI: 10.7241/ourd.2020e.94
Cite this article: Kettani F-Z, Hali F, Baline K, Marnissi F, Chiheb S. Cutaneous lymphangitis carcinomatosa of breast cancer: a series of 7 cases. Our Dermatol Online. 2020;11(e):e94.1-e94.5.
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Cutaneous metastases of breast cancer represent a rare secondary location with a poor prognosis. Cutaneous lymphangitis carcinomatosa (CLC) secondary to breast neoplasia is a rare form of cutaneous metastasis. We report a series of 7 cases in a Moroccan population.
Materiel and Methods: We identified all patients with breast cancer who were referred to our service for a skin eruption that was later diagnosed as a CLC from February 2018 to October 2019.
Results: Seven patients, aged 54 years on average, were followed for breast cancer. After a period of 1 to 5 years, they developed cutaneous lesions that was resistant to symptomatic treatment. Clinical examination found: erysipelatoid eruption (4 cases), lymphedema with erythema and swelling (3 cases), erythematous maculopapular lesions with ulceration and crusts (3 cases), papulo-nodular eruption (2 cases), enlarged breast (1case) and an indurated plaque (1 case). Histological examination revealed cutaneous metastasis of breast cancer with dermal carcinomatous lymphangitis in all cases. The extension assessment found other solid metastases in 5 cases. Four patients died after 3 to 8 months after making the diagnosis of CLC.
Conclusions: Only 30 cases of CLC secondary to breast neoplasia were found in a review of the literature carried out in 2012. Our series is, presumably, the largest series reporting CLC cases of breast cancer. Clinically, the eruption can take various aspects, often confusing. A skin biopsy must be done in front of any suspicion of CLC to confirm the diagnosis and thus adapt the management. The prognosis is generally pejorative with early deaths.
Key words: Cutaneous lymphangitis carcinomatosa; Breast cancer; Skin metastases
INTRODUCTION
Cutaneous metastases of breast cancer represent a rare secondary location with a poor prognosis. Cutaneous lymphangitis carcinomatosa (CLC) secondary to breast neoplasia is even rarer. Its clinical presentation may be polymorphous and often confusing [1]. We report a series of 7 cases of CLC of breast cancer, with different clinical presentations.
METHODS
We identified all patients with breast cancer who were referred to our department for a skin eruption that was later diagnosed as a CLC from February 2018 to October 2019.
We collected clinical and epidemiologic data, the type of breast cancer, the treatments received, the time interval between the diagnosis of breast cancer and the appearance of cutaneous lesions. Skin biopsies were done in all cases. Once the diagnosis of CLC was confirmed, we performed an extension assessment in all our patients. The follow-up of the patients continued through the oncology unit.
RESULTS
A total of 7 patients were diagnosed with CLC. The average age was 54, with extremes ranging from 42 to 68.
Initially, breast cancer was treated by surgery (mastectomy in 5 patients, lumpectomy in 2 patients) chemotherapy and radiotherapy in all patients. Two patients were also treated with hormone therapy.
After a period of 1 to 5 years, patients have developed progressive skin lesions for which they were referred to our dermatology department. These lesions evolved for 5 months on average, were resistant to the different symptomatic treatments previously received (topical corticosteroids, oral and systemic antibiotics, antiviral treatment). The accompanying symptoms were pain, pruritus and sensation of burning, depending on the case
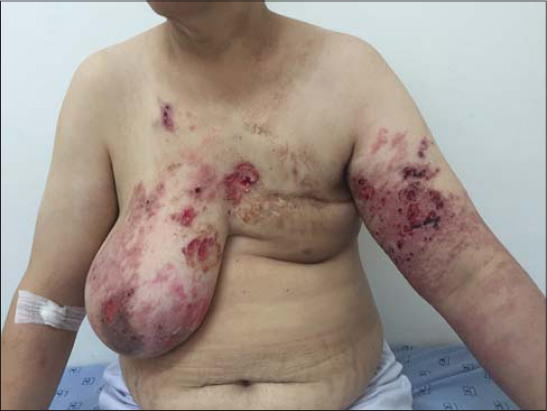
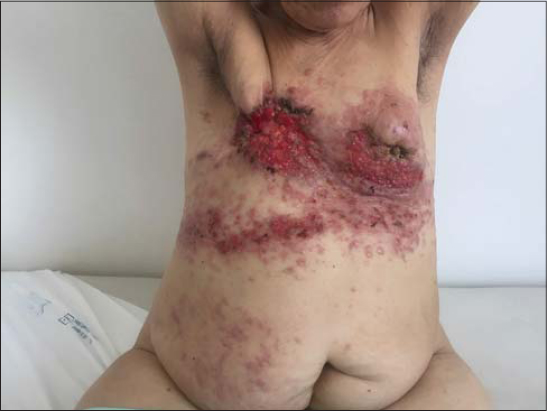
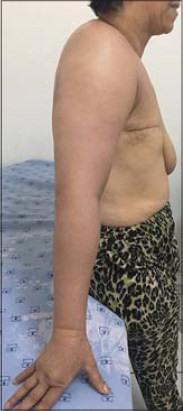
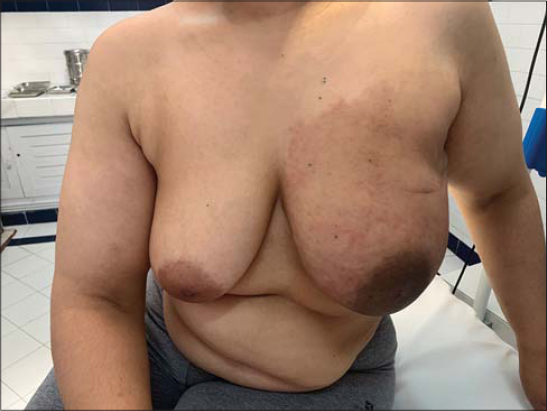
The skin eruption was variable (Figs. 1 – 4). We encountred different clinical presentations: an erysipelatoid rash in 4 patients, a lymphoedema with erythema and swelling in 3 patients, erythematous maculopapular lesions with ulcerations in 3 patients, papulo-nodular eruption in 2 patients, an induration in 1 patient, an enlarged breast in 1 patient. These lesions were located on the pathological breast region (5 cases), on the upper limb (3 cases), and extended on the contralateral breast (3 cases), thorax and abdomen (2 cases) and scapular region (1 case).
Some patients had a combination of multiple lesions (Table 1).
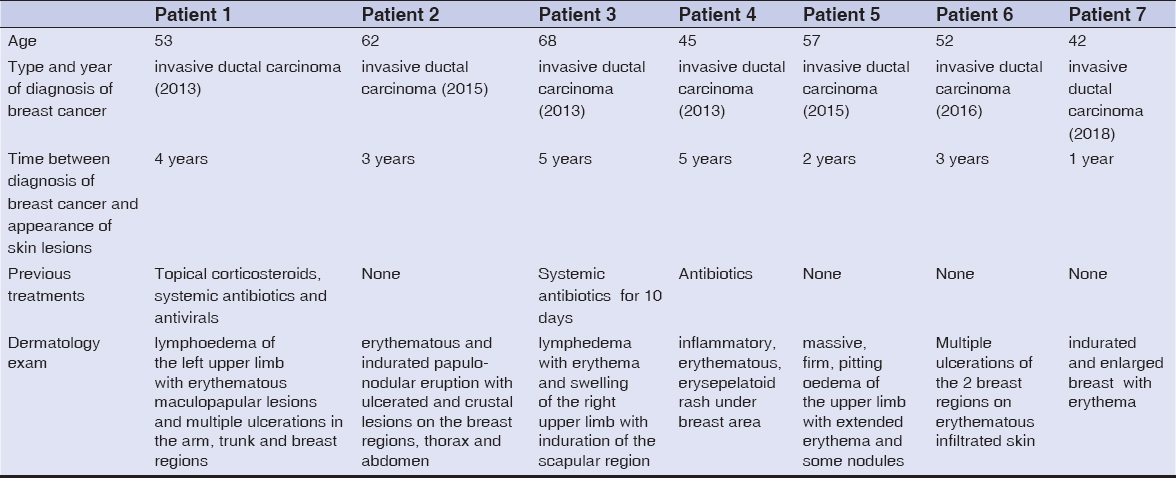 |
Table 1: Age of patients, type and year of diagnosis of breast cancer, time between diagnosis of breast cancer and appearance of skin lesions, previous treatments and dermatology exam |
A skin biopsy was performed in each case. It showed in all patients CLC secondary to breast cancer (Fig. 5). Further examination, including magnetic resonance imaging and computerized tomography scans were performed. They revealed solid metastasis in 5 patients. These metastases were cerebral, pulmonary, hepatic and osseous.
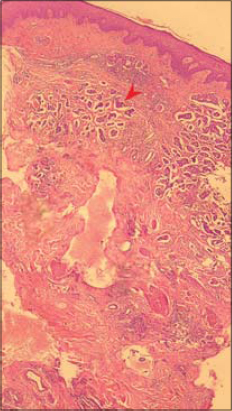 |
Figure 5: Infiltration of the dermal lymphatics by metastatic breast carcinoma (see arrow) (haematoxylin-eosin stain; magnification x100). |
After making the diagnosis of CLC, the patients were referred to an oncology center for management. 4 patients died after 3 months, 4 months, 5 months and 8 months respectively of the diagnosis.
DISCUSSION
CLC secondary to breast neoplasia is a rarely described entity [1]. A review of the literature (cases published in Pubmed since 1986 and review of bibliographic references of selected articles) found only 30 cases of CLC [2]. Our series is, to our knowledge, the largest series reporting CLC cases of breast cancer.
The skin represents the 12th site of metastases for deep cancers. Breast carcinoma is by far the most common primary neoplasm resulting in skin involvement [3].
Cutaneous metastases (CM) are present after breast carcinoma in about 23.9% of patients, often involving the chest and abdomen and manifesting on average 5 years after surgical removal of the first malignancy [4]. Typical CM are due to a dense infiltrate of neoplastic cells in the dermis or the hypodermis and are described as unique or multiple dome-shaped, reddish, painful nodules or masses with a moderate to firm consistency, this aspect accounting for 70–80% of cases [5].
However, several clinical features have been reported in the literature, including telangiectatic carcinoma, erythema-like, erythema annulare centrifugum- like, morphea-like, erysipelas-like, dermatofibroma- like, herpes-zoster-like, and alopecia like lesions [4].
Intravascular metastases of breast carcinomas are rare and may spread through dermal blood vessels of dermal lymphatic vessels [6]. Cutaneous lymphangitis carcinomatosa is a rare presentation of skin metastases. It accounts for approximately 5% of all cutaneous metastases. It is characterized by an occlusion of dermic lymphatic vessels by neoplastic cells [4,5]. Various terms have been used in the literature such as lymphangitis carcinomatosa, erysipelas carcinomatosa and metastasis cutis [7].
Clinically, the eruption is polymorphous, able to mimic different dermatoses; thus delaying the diagnosis and proper management of the lesions. It usually presents as an erythematous, tender area closely resembling an acute infectious process, i.e., erysipelas or cellulitis. However, CLC is not associated with fever, chills, and leukocytosis. Furthermore, CLC shows no response to antibiotic therapies [4,5]. Three of our patients were treated with antibiotics for suspecting erysipelas before correcting the diagnosis with skin biopsy. CLC may also occur as nonspecific eczematiform lesions, angiomatous or sclerodermiform lesions, or in the form of localized lymphedema [2,7,8]. When the diagnosis of lymphoedema is made in patients previously treated for malignancy, the physician must consider whether recurrence is the cause or lymphedema is a complication of the initial cancer treatment. Secondary lymphoedema due to malignant infiltration should be considered particularly when lymphedema develops quickly, is constantly present, dilated veins are visible and patients are complaining of intolerable, intense pain [7].
In the series of 4 cases of Hariz et al, they reported several aspects (papulo nodular and erosive rash, erysipelas, radiation-induced lichen, eczema) [1]. Sometimes, different clinical aspects may exist in the same patient; as was the case for our third patient who presented with both lymphoedema and swelling of the upper limb and induration of the scapular region. Prat and al have also noted in the same patient lymphedema of the root of the limb, with eczematiform, erysipelatoid and angiomatous lesions elsewhere [2].
Our series of 7 patients is a good illustration of the clinical diversity of CLC. The clinical presentations were different from one patient to another. We noted several types of lesions that was diagnosed as CLC, namely an indurated papulo-nodular eruption with ulcerated and crustal lesions, lymphedema with erythema and swelling, erysepelatoid rash and indurated plaque.
CLC has been reported in the literature in association with several malignancies, including lung, breast, ovarian, pancreatic and rectal cancers [2,4,6].
The treatment is that of the primary cancer [6]. Though, clinical regression of lymphangitic carcinomatosis has been reported, e.g., with intralesional injection of interleukin-2 [9] or with topical Imiquimod 5% [10].
The prognosis is generally pejorative with early deaths [1,6,10].
Similar to the other reports in the literature, four of our patients died shortly after the appearance of the CLC. This indicate that lymphogenic spread in the skin might be a sign of lymphogenic metastases elsewhere and possibly also a marker of rapid tumour progression.
CONCLUSION
This report illustrates the clinical polymorphism and the diagnostic difficulty of CLC.
In a patient with a history of cancer, we stress that every cutaneous lesion should alert the clinician to the possibility of a cutaneous metastasis revealing a cancer recurrence. An early histological examination should be performed looking for that entity that may be under diagnosed.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent
Informed consent was obtained from all patients for being included in the study.
Copyright by Fatima-Zahra Kettani, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
REFERENCES
1. Hariz W, Boudaya S, Soua Y, Mseddi M, Turki H. Lymphangite carcinomateuse cutanée secondaire àun cancer du sein:àpropos de quatre cas. Ann Dermatol Vénéréol. 2011;138:A214.
2. Prat L, Fardet L, Chouaid C, Toledano C, Mauhin W, Kettaneh A, et al. Lymphangite carcinomateuse cutanée. Cas clinique et revue de la littérature. Rev Méd Interne. 2012;33:S185.
3. Marcoval J, Moreno A, PeyríJ. Cutaneous infiltration by cancer. J Am Acad Dermatol. 2007;57:577-80.
4. Paolino G, Panetta C, Didona D, Donati M, Donati P. Folliculotropic cutaneous metastases and lymphangitis carcinomatosa:when cutaneous metastases of breast carcinoma are mistaken for cutaneous infections. Acta Dermatovenerol Croat ADC. 2016;24:154-7.
5. Prat L, Chouaid C, Kettaneh A, Fardet L. Cutaneous lymphangitis carcinomatosa in a patient with lung adenocarcinoma:Case report and literature review. Lung Cancer. 2013;79:91-3.
6. Steff M, Marinho E, Crickx B, Descamps V. Un érysipèle récalcitrant. Imag Dermatol. 2011;IV:2.
7. Damstra RJ, Jagtman EA, Steijlen PM. Cancer-related secondary lymphoedema due to cutaneous lymphangitis carcinomatosa:clinical presentations and review of literature:Cancer-related secondary lymphoedema. Eur J Cancer Care (Engl). 2010;19:669-75.
8. Litaiem N, Jones M, El Fekih N, Khaled A, Rammeh S, Zermani R, et al. Métastases cutanées du cancer du sein:deux présentations trompeuses et rares. Ann Dermatol Vénéréol. 2013;140:S111.
9. Hamamoto Y, Nagai K, Ichimiya M, Yamamoto K, Kinoshita E, Muto M. Regressive effect of intralesional injection of a moderate dose of recombinant interleukin-2 on carcinoma erysipeloides from gastric carcinoma. Clin Exp Dermatol. 2001;26:42-4.
10. Jaeger T, Zirbs M, Andres C, Gutermuth J, Ring J, Rodriguez R, et al. Lymphangitic carcinomatosis of colon carcinoma:a sign of poor prognosis:A memorable patient. Clin Exp Dermatol. 2012;37:937-9.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-4789-6652 http://orcid.org/0000-0003-4789-6652 |



Comments are closed.