The efficacy and safety of diphenylcyclopropenone solutions in propylene glycol and isopropanol. A comparative study of two formulas used for the treatment of 100 patients with alopecia areata
Katarzyna Borowska1, Tomasz Wasyłyszyn2
1Department of Histology and Embryology with Experimental Cytology Unit, Medical University of Lublin, 11 Radziwiłłowska, 20–080 Lublin, Poland
2Military Institute of Medicine in Warsaw, Department of Dermatology, Szaserów 128, 04-141 Warsaw, Poland
Corresponding author: Prof. Katarzyna Borowska, E-mail: k_borowska@wp.pl
Submission: 24.10.2017; Acceptance: 27.10.2017
DOI: 10.7241/ourd.2017e.13
How to cite this article: Borowska K, Wasyłyszyn T. The efficacy and safety of diphenylcyclopropenone solutions in propylene glycol and isopropanol. A comparative study of two formulas used for the treatment of 100 patients with alopecia areata. Our Dermatol Online. 2017;8(4e):e4.
ABSTRACT
Alopecia areata (AA) is a T cell-mediated autoimmune disease involving hair follicles characterized by hair loss. 2,3-diphenylcyclopropenone (DCP) is a topically administered drug intended for treating AA. The study investigates an efficacy and safety of DCP for the purpose of the treatment of AA. It presents a comparative study of two formulas: DCP in propylene glycol and DCP in isopropanol. While the treatment efficacy in both groups was very simmilar, the tolerance of the DCP in isopropanol was better than DCP in propylene glycol. Authors indicate the potential clinical applications of latter formula.
Key words: diphenylcyclopropenone, DCP, alopecia areata, AA, propylene glycol, isopropanol
INTRODUCTION
Alopecia areata (AA) is a T cell-mediated autoimmune disease involving scalp and body hair follicles leading to hair loss. Occurrence in the population AA ranges from 0.7% to 3.8% [1,2]. Etiopathogenesis of AA is unknown but evidence exists to support genetic, immune and environmental factors [1-3]. 2,3-diphenylcyclopropenone (DCP) was used as an immune-modulating therapeutic factor for AA for several decades [4]. Majority of authors used DCP solutions in propylene glycol [3] or acetone [5]. In spite of the different results authors investigated the stability of DCP solutions in various solvents (acetone, ethanol, propylene glycol and isopropanol) to obtain basic pharmacokinetics including chemical stability which was lacking in the literature [6,7]. Results of these investigations revealed the potential usefulness of isopropanol as a solvent which maintains relatively good stability of DCP while being cosmetically acceptable. It also showed that DCP solutions in acetone are very unstable. The authors next report was the clinical one [8] showing that DCP solutions in isopropanol may indeed have potential in the treatment of AA. The tolerance of the DCP in isopropanol was significantly better than DCP in propylene glycol while clinical efficacy was maintained [8]. The aim of this study was to assess the efficacy and safety of DCP for the purpose of the treatment of AA. The above mentioned study dealt with two groups of 20 patients, treated with DCP in glycol and DCP in isopropanole respectively. It was first pilot study to indicate that DCP in isopropanol formula may have the appliance in the future. The present study is a comparative study of the same formulas-DCP: propylene glycol and isopropanol (isopropyl alcohol) but on larger groups to discuss the previously found data in spite of new ones.
MATERIAL AND METHODS
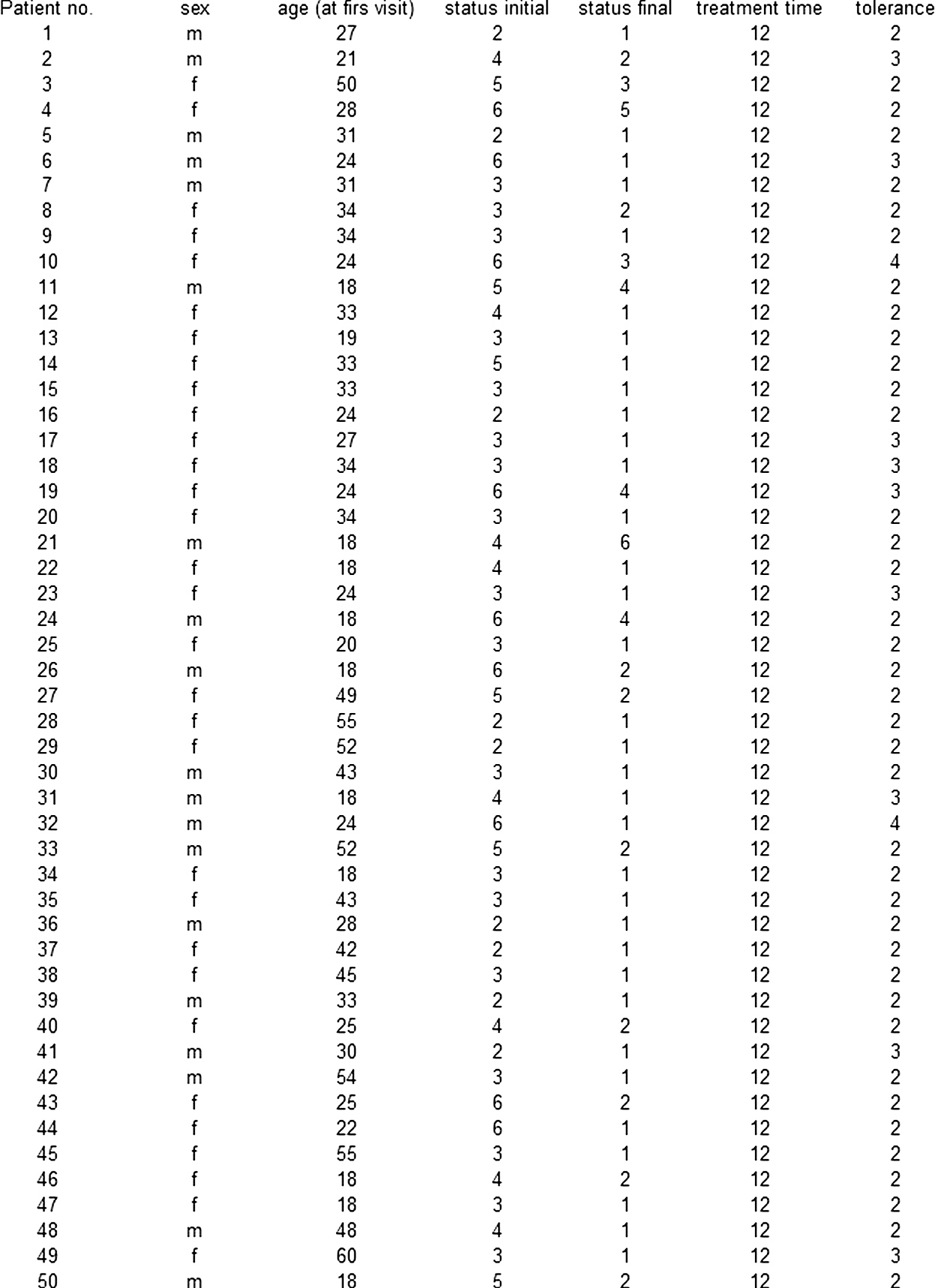
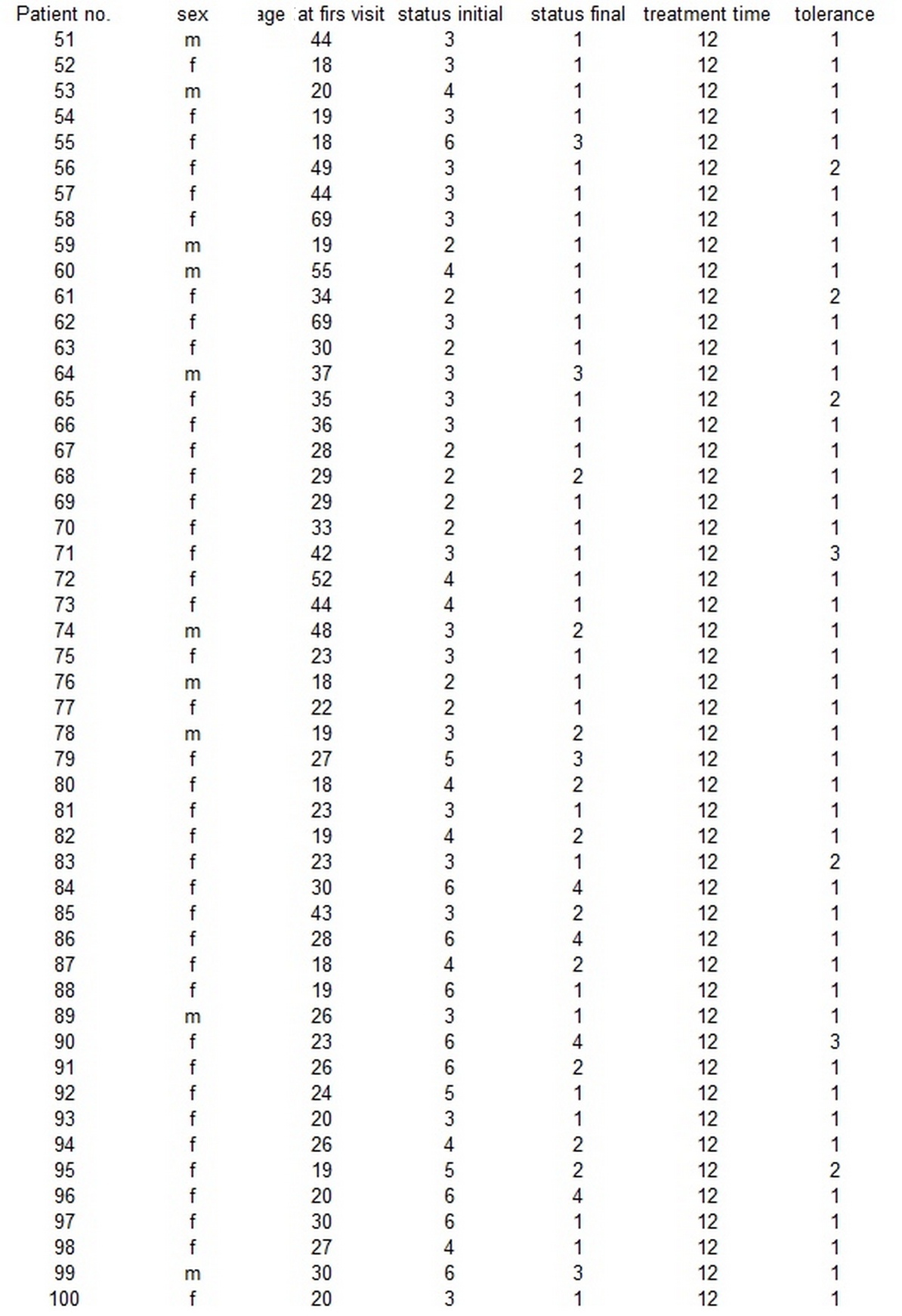
After having an agreement with ethics committee (22/WIM/2016), the authors arranged two groups of patients with AA, 50 patients in each. Groups were standardized using age at the beginning of a treatment and clinical status at the beginning of the study. Group I was treated with DCP in propylene glycol while group II with DCP in isopropanol. The demographic data of the patients are summed up in table 1 and table 2.
Solutions were made of 98% 2,3-diphenylcyclopropenone chemical by Sigma Aldrich (No. 177377). The solutions of 3% DCP were prepared in both solvents respectively. All solutions were kept in the refrigerator at the temperature of 4°C according to data obtained from stability studies. From these solutions, the solutions of 0.5%, 0.3%, 0.2%, 0.1% and 0.05% were made in each solvent in order to perform the proper treatment. In both groups on the first visit, 3% DCP was applied to bald areas on the top of the scalp in order to obtain sensitization. Here it is important to mention, that no matter if patient was treated with DCP in propylene glycol or DCP in isopropanol during proper treatment, the sensitization was always performed with 3% DCP in propylene glycol. Areas near the neck and ears were avoided to prevent serious irritation – even if bald patches were there. Patients were instructed to avoid situations which may lead to sweating (like physical exercises) for the next two days. During 24 to 48 hours after application, a reaction occurred including itching, redness, edema and the presence of vesicles with serous liquid. After 2 days, patients were told to wash their scalps and the reaction gradually faded. Further applications took place during 1 week intervals. The DCP concentration was at that time between 0.05% and 0.5% (usually about 0.1%), depending on the intensity of the reaction after sensitization. Patients were given the tube of strong corticosteroid ointment (clobetacole propionate 0.05%) and were advised to use it if allergic reaction to DCP occurs on the body parts. All the patients were observed for 12 months. To assess the grade of hair amount authors used their own invented scale that was used in previous studies (3,8), therefore the citated exactly like before. Grade 1 of this scale indicated no hair loss or complete hair regrowth, grade 2 showed bald area below 20% of the scalp surface, grade 3- represented bald area between 21 and 50% of the scalp surface, grade 4 – had a bald area between 51 and 80% of the scalp area, grade 5 – reflected a bald area between 81 and 99% of the scalp surface and finally, grade 6-total 100% hair loss (alopecia totalis). In the discussion grades 1 and 2 are considered as “cosmetically acceptable”. The assessment was made twice: at the beginning and at the end of the observation. In addition, the tolerance of the treatment was assessed by patients using the other 6 grade scale, in which grade 1 indicated perfect tolerance, while grade 6 indicated complete lack of tolerance (Table 3). For the purpose of statistical analysis Kruskal-Wallis rank sum test and Shapiro-Wilk normality test were used.
RESULTS
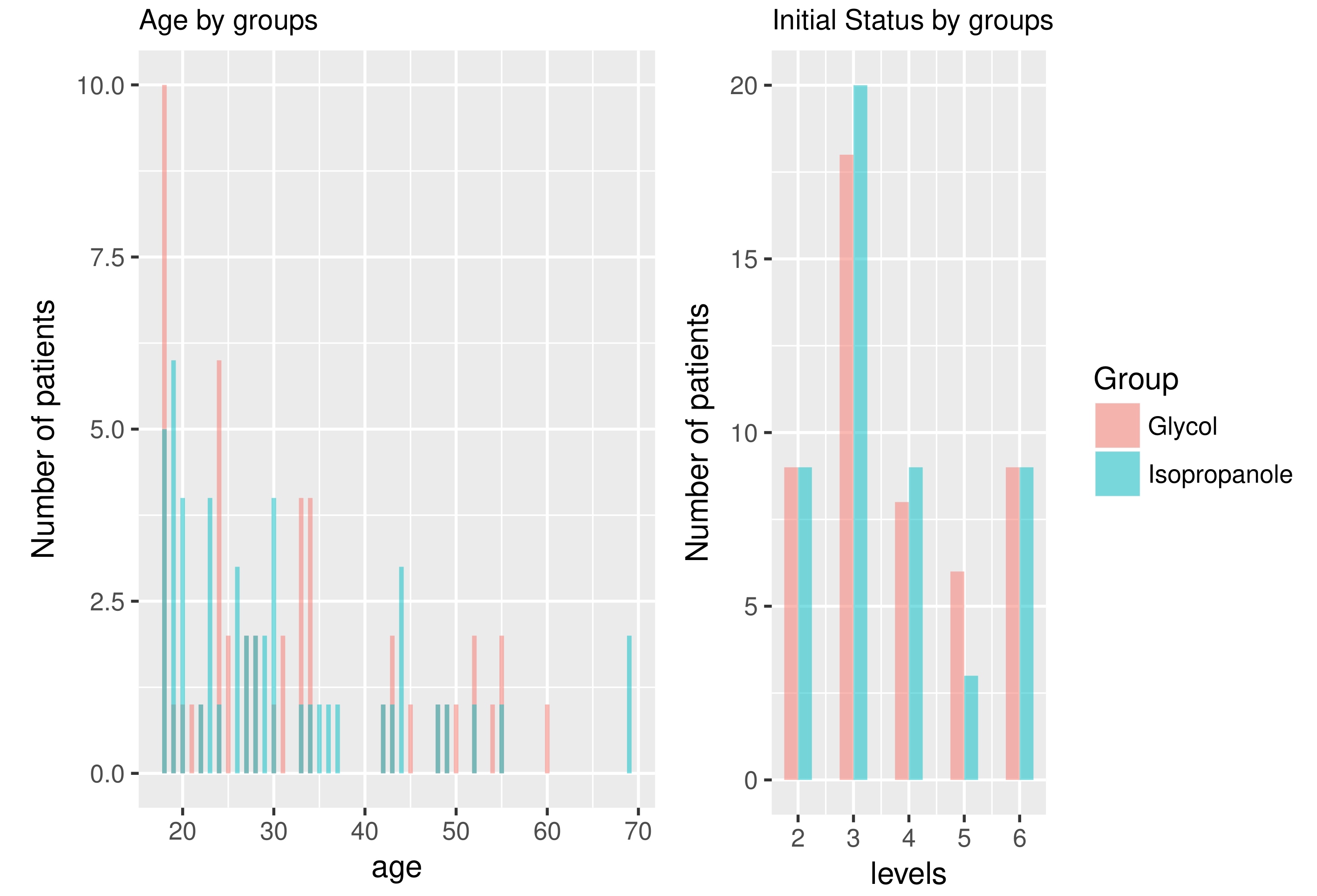
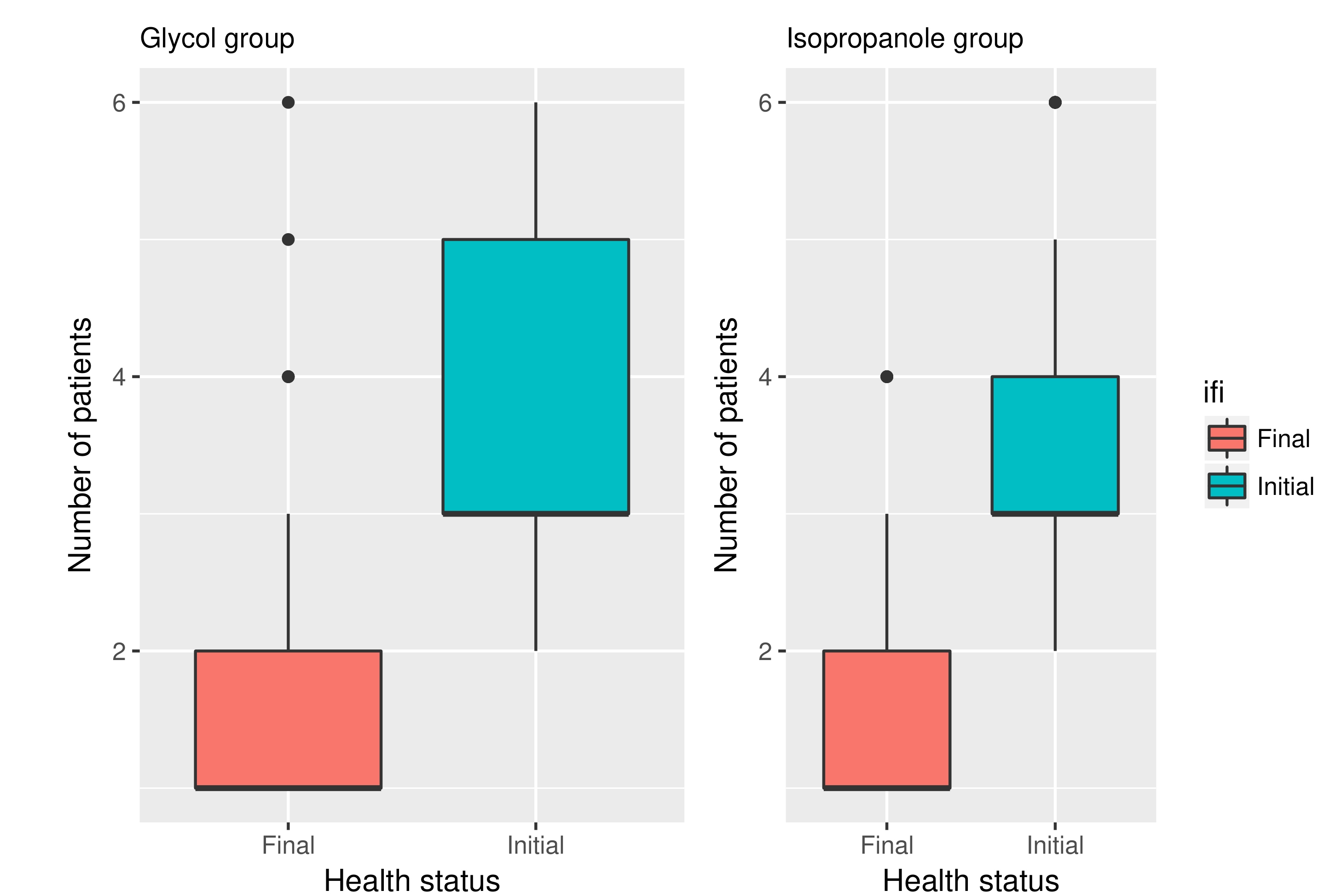
Standardization was good and there was no statistically significant difference between the groups taking into consideration their age and initial status of the disease (Fig. 1) Complete hair regrowth was achieved in 68% and 64% of patients in groups I and II respectively (initial amount of complete regrowth was zero obviously). All patients responded to the study with at least partial hair regrowth. Satisfactory improvement after the treatment was achieved in both groups I and II respectively with 85% and 84% of patients with more than 80% hair regrowth (grades 1 and 2 in the hair loss scale). By comparison initial values (of patients with more than 80% hair) in both of these groups were 18%. Calculated treatment efficacy in both groups was statistically important (Fig. 2). Majority of the patients noted an improvement by two degrees in hair loss scale described above. Differences in treatment's efficacy between groups were not statistically important though.
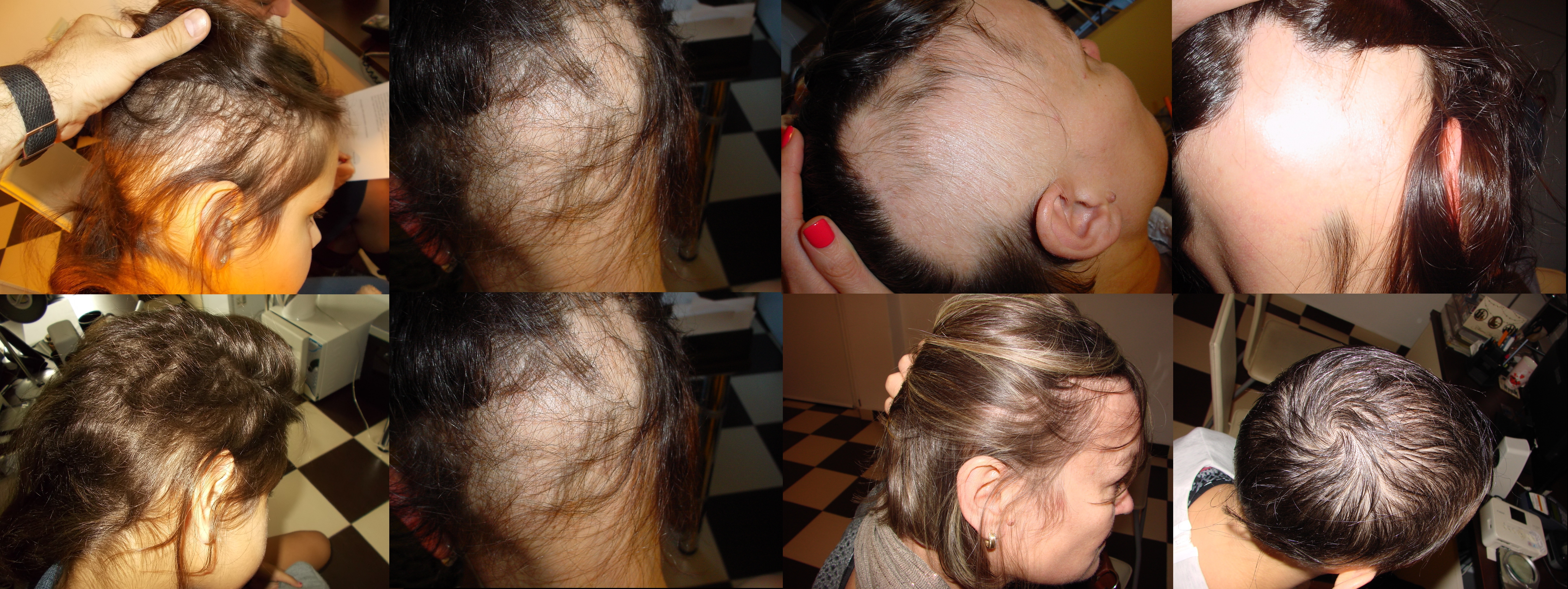
Tolerance was generally good but better in a group treated with DCP in isopropanol. While patients treated with DCP in propylene glycol usually reported tolerance level between 2 and 3 with mean tolerance of 2.26, those treated with DCP in isopropanol reported tolerance grade 1 to 2 (mean 1.18). This means that group IL (treated with DCP in isopropanol) described treatment as perfectly tolerable or experiencing mild side affects that do not disturb routines. However, in group II allergic reactions affecting the body were reported more frequent; usually disappearing during two-three days but unpleasant. The itching patches on the upper body parts sometimes required the usage of corticosteroid ointment for few days. None of the patients had to withdraw the treatment because of the side effects. Figure 3 presents clinical status before and after the treatment of four chosen patients: two treated with DCP in propylene glycol and two with DCP in isopropanol.
The mechanism of action of DCP in human skin has not been clearly defined. DCP is a hapten that induces DTH (delayed-type hypersensitivity) reactions involving a cytokine response and local infiltration of T-cell subpopulations, resulting in contact dermatitis. DCP induces change the perifollicular CD41/CD81 T-lymphocyte ratio, apoptosis of perifollicular lymphocytes and modulate proinflammatory cytokines [3,10].
Authors hypothesize once again that it is crucial to understand that DCP in contact with large amounts of water decomposes quickly to diphenylacetylene, which might be responsible (probably even more than DCP itself) for serious irritation during treatment [6,7]. While the stability of DCP in both isopropanol and propylene glycol is very similar it is supposed, that solutions in glycol, due to their sticky consistence provoke skin sweating and thus water present in the sweat decomposes DCP quickly. Of course DCP eventually decomposes anyway but it is the fact that propylene gycol keeps the decomposition product (diphenylacetylene) close to the skin's surface that is responsible for formula's worse tolerance. In contrary, isopropanol evaporates from the skin surface very rapidly (almost as quick as ether), leaving pure DCP on it's surface. Therefore authors suggest that DCP solutions in isopropanol may have an appliance in the future as a new formula designed for the treatment of AA.
CONCLUSION
REFERENCES
1. Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177-88.
2. Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part II. Treatment. J Am Acad Dermatol. 2010;62:191-202.
3. Wasyłyszyn T, Kozłowski W, Zabielski SL. Changes in distribution pattern of CD8 lymphocytes in the scalp in alopecia areata during treatment with diphencyprone. Arch Dermatol Res. 2007;299:231-37.
4. van der Steen PH. Boezeman JB. Happle R. Topical immunotherapy for alopecia areata: re-evaluation of 139 cases after an additional follow-up period of 19 months. Dermatology. 1992;184:198-201.
5. Singh G, Lavanya M. Topical Immunotherapy in Alopecia Areata. Int J Trichol. 2010;2:36-9.
6. Borowska K, Wasyłyszyn T. Studies on stability of 2,3-diphenylcyclopropenone in contact with water and aqueous NaCl solutions. Conclusions for the purpose of topical therapy of patients with alopecia areata. Acta Poloniae Pharm Drug Res. 2017;74:459-64.
7. Wasyłyszyn T, Borowska K. The stability of solutions of 2,3-diphenylcyclopropenone in various solvents. A novel formula – diphenylcyclopropenone in isopropanol may be useful in topical therapy of patients with areata. Acta Poloniae Pharm Drug Res. 2016;73:1455-60.
8. Wasyłyszyn T, Borowska K. Efficacy and tolerance of 2,3-diphenylcyclopropenone in propylene glycol versus 2,3-diphenylcyclopropenone in isopropanol-a novel formula designed for the treatment of alopecia areata. Our Dermatol Online. 2017;8:17-21.
9. Chiang K, Atanaskova Mesinkovska N, Amoretti A, Piliang MP, Kyei A, Bergfeld WF. Clinical efficacy of diphenylcyclopropenone in alopecia areata: retrospective data analysis of 50 patients. J Am Acad Dermatol. 2014;71:595-7.
10. Herbst V, Zöller M, Kissling S, Wenzel E, Stutz N, Wenzel E, Stutz N, Freyschmidt-Paul P. Diphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytes. Eur J Dermatol. 2006;16:537-42.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.

Comments are closed.