A clincodermoscopic evaluation of chronic diffuse hair loss among females in a Tertiary Care Center
Sathish Shankar, Raghavendra Kalasapura Ramegowda , Bangaru Hanumaiah, Ashok Kumar Prasad
, Bangaru Hanumaiah, Ashok Kumar Prasad
Department of Skin and VD, MMCRI, Mysore, India
Corresponding author: Raghavendra Kalasapura Ramegowda, MD
How to cite this article: Sathish S, Raghavendra KR, Bangaru H, Prasad AK. A clincodermoscopic evaluation of chronic diffuse hair loss among females in a Tertiary Care Center. Our Dermatol Online. 2022;13(e):e50.
Submission: 28.05.2022; Acceptance: 21.07.2022
DOI: 10.7241/ourd.2022e.50
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Diffuse alopecia in females is a common complaint and still a major challenge in the practice of dermatology. Female androgenetic alopecia (FAGA), chronictelogen effluvium (CTE), and diffuse alopecia areata(AA) are its three main causes for diffuse hair loss. It is often difficult one from the other and clinical data, physical examination, biochemical tests, dermoscopy, and some cases biopsy may be needed for a definitive diagnosis.In this study, scalp dermoscopy as a main tool used to differentiate between different causes of hair loss and definitive role of dermoscopy in the treatment follow up.
Objectives: Our aim is to evaluate and determine dermoscopic findings of different causes of diffuse alopecia that can be used as the clinical indicator of the disease. Another aim is to study the correlation of haemoglobin, S. ferritin and TSH levels in different causes of diffuse alopecia in females.
Methods: Total50 patients were enrolled for this prospective study and was conducted for a period of 6 months. Detailed history, Clinical examination, Dermoscopic evaluation, complete hemogam, S. ferritin and Thyroid profile was done.
Results: Most of them (66%) were between age group 18 to 30. Out of total cases 31 cases were TE, 9 cases were FAGA, 7 cases of diffuse Alopecia. Dermoscopic findings seen were yellow dots in 41 patients, thinning of hair in 33 patients, vellus hairs in 14 patients, peripilar sign in 12 patients, hair diameter diversity more than 20 % (HDD) in 8 patients, exclamation mark hair in 7 patients, honey comb pigment pattern and loss of follicles in 3 patients each.
Conclusion: In TE cases most common dermoscopic findings were Yellow Dots, In FPHL most common and specific finding was HDD > 20%. A thorough dermascopic evaluation and follow up is must for different patterns of hair loss among females.
Key words: Female androgenetic alopecia; Chronic Telogen Effluvium; Dermoscopy; Hair diameter density
INTRODUCTION
Hair loss in females is a most common complaint and amajor challenge in the routine practice of dermatology. Female androgenetic alopecia (FAGA) or Female pattern hair loss (FPHL), chronictelogen effluvium (CTE), and diffuse alopecia areata(AA) are its three main causes. Most of the time, it isverydifficult to distinguish the cause for hair loss from one another. A clinical data, laboratoryfindings, physical examination, Dermoscopy, and some cases biopsy may be needed for a definitivediagnosis [1–3]. In this study,scalp dermoscopy is done to differentiate between different causes of diffuse alopecias in females.
Hair loss is the most common hair problem and a prompt diagnosis of the type of alopecia may sometimes be extremely challenging. Methods commonly used to investigate are bedside tests like hair pull test, hair count test, trichoscopic analysis, biochemical tests like complete hemogram, thyroid profile, Serum ferritin, Serum Vitamin D3levels, sometimes trichogram and scalp biopsy may be indicated [4].
Non anaemic iron deficiency was suggested as one of the etiological factor for diffuse hair loss in women in both CTE and FAGA [5]. Assessment of serum ferritin levels and complete hemogram is therefore generally recommended as part of the routine investigation. Studies have shown there is strong association between hair loss and thyroid disorders; hence women suffering from recalcitrant hair loss should undergo thyroid function tests to reveal subclinical thyroid disorders [6].
Dermoscopy of the scalp now a day has been increasingly used in routine practice. The available dermascopic methods are traditional dermatoscope, dermatoscope with polarized light, videodermatoscope with polarized light, and their association with a computerized analysis. When dermoscopy used without immersion it will be useful for analyzing tertiary structures of the scalp such as vellus hair, irregularities, sweat pores, sebum secretion, and exudation. Dermoscopy can be used in evaluation or therapy follow-up, with an area being permanently marked with a small tattoo and sometimes with fixed points also. Various trichoscopic patterns observed in alopecias are: Yellow dots, black dots, white dots, dystrophic hairs, Hair shaft diameter variation of>20% hair shaft, Peripilar halo, peripilarcasts [7]. The dermascopic examination for scalp for all types of hair loss among females is a must. So in this study we have evaluated the role of dermascopy in hair loss evaluation.
METHODOLOGY
All female patients with hair loss between the age group of 18 to 50 years attending the out-patient clinic of the Department of Dermatology, Venereology and Leprosy at Mysore Medical college and research institute, Mysore, will be considered for the study. Consent will be taken from the individual patients to include them in the study. A detailed history of the patient including age, sex, address, education, occupation; duration and extent of hair loss, history of having used any topical agent or other methods of treatment will be noted. History of intake of any systemic drugs will also be recorded.
The diagnosis of FPHL, TE, and CTE was made based on the history and clinical presentation at the time of first visit of the patient. Patients giving preceding history of stress in the past 2–3 months and positive hair pull test were diagnosed as TE. Patients presenting with hair thinning over central/bitemporal areas and negative hair pull test were diagnosed as FPHL. Patients with hair fall for >6 months without any widening of central parting and positive hair pull test were diagnosed as CTE. In patients having overlapping features, a diagnosis was made on the basis of most prominent clinical signs. We did not include minors (<18 years) and pregnant females [8]. Detailed clinical examination, Dermoscopic analysis and biochemical tests like CBC, TFT, Serum Ferritin levels was done and analysed.
RESULTS
Out of total 50 cases, the mean age was 28.34 years. 19 patients were in age group 18 to 25, 14 in the age group 26 to 30, 8 in the age group 31 to 35, 3 in the age group 36 to 40 and 6 in the age group 41 to 45. Most of them (33 patients, 66%) were between age group 18 to 30.
Out of 50 cases 31 (61%) cases were Telogen effluvium; of which 20 cases of acute Telogen effluvium and 11 cases of chronic Telogen effluvium, 9 (19%) cases were Female androgenetic alopecia, 7 (14%) cases of diffuse alopecia areata and 3 (6%) cases of diffuse Discoid lupus erythematosus of scalp. In our study Telogen effluvium was the most common cause of diffuse hair loss followed by FAGA, Diffuse AA.
In our study least value of Hb observed is 7.5 gm/dl and highest of 14.1 gm/dl mean value of 11.56 gm/dl which less than the normal limit. 29 patients had Hb values less than 12 gm/dl of which 17 patients were TE, 5 patients were FPHL, 5 patients were AA and 2 were DLE. Mean ± SD of Hb levels in TE is 11.4± 1.76 and in FPHL it is 12 ± 1.3, P value calculated using un-paired T test is 0.35 (p >0.05) hence the difference between Hb levels in TE and FPHL is not statistically significant.
In our study least value of S. ferritin observed is 3.9 ng/ml and highest of 79.3ng/ml (range 3.9 to 79.3ng/ml), mean of 30.21 ng/ml which is towards the lower limit, 8 patients had very low ferritin levels (less than 10ng/ml) all of them had TE. Mean value± SD of ferritin levels in TE cases is 26± 20.05 ng/ml, in FPHL it is 33.6 ± 28.04 ng/ml, P value calculated using unpaired T test was 0.36 (p value > 0.05) hence the difference between S.ferritin levels among TE & FPHL was not statistically significant. In Difuse AA the mean was 36.85ng/ml and in DLE it is 47.53ng/ml (Table 1).
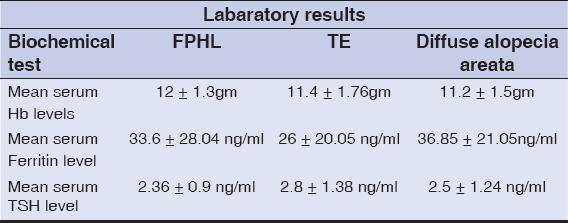 |
Table 1: Laboratory results of three types of alopecia |
In our study least value of TSH observed was 0.49 mIU/ml and highest of 7.13 mIU/ml, mean value is 2.7 mIU/ml which is within normal range, 5 patients had TSH values above 5 mIU/ml and all these 5 patients had Telogen effluvium in rest other conditions TSH was normal indicating association of hypothyroidism with TE. Mean± SD of TSH value among TE cases is 2.8± 1.38 and in FPHL it is 2.36± 0.9, P value is 0.38 which is not statistically significant.
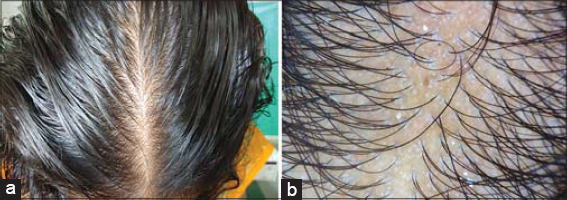
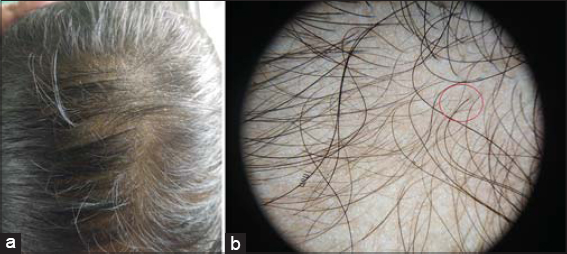
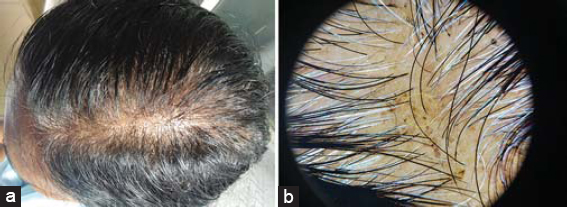
Dermoscopic findings seen were yellow dots in 41 patients, thinning of hair in 33 patients, vellus hairs in 14 patients, peripilar sign in 12 patients, hair diameter diversity more than 20 % (HDD) in 8 patients, exclamation mark hair in 7 patients, honey comb pigment pattern and loss of follicles in 3 patients each. Dermoscopic findings seen in different patterns of hair loss are tabulated (Table 2).In total 31 cases of telogen effluvium; yellow dots in 26 patients (83%) (Figs. 1a and 1b). Thinning of hairs in 18 (58.1%), peripilar sign in 8 (25.8%) and vellus hairs in 4 (12.9%). In 9 patients of FPHL; HDD in 8 (88.8%), yellow dots in 8 (88.8%) and thinning of hairs in 8 (88%) (Figs. 2a and 2b). In 7 patients of diffuse AA; exclamations mark hairs in 7 (100%), vellus hairs in 7 (100%), yellow dots in 6 (85.7%) and thinning of hairs in 5 (71.4%) and honey comb pigment pattern (Figs. 3a and3b).
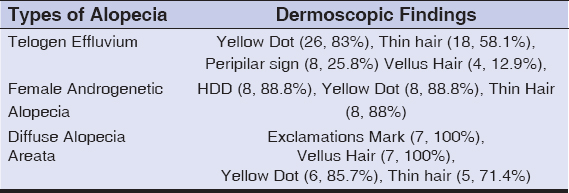 |
Table 2: Prominent dermoscopic features in different patterns of alopecia |
DISCUSSION
In general, FPHL has its onset during the reproductive age group. The most common age group between 25-40 years experience the hair loss. The second peak of incidence occurrence at menopause, between 50 and 60 years of age. In our study out of 50 patients mean age was 28.34, in another study by Ramatulasi et al mean age was 33 [9], in another study by Ummiti et al it was 37 [10], which was slightly higher than our observation. In a study by Shilpashree et al the mean age was 28.4 [11], and by Tandon et al it was 26.1 both were comparable with our results [12].
Normal range of Hb in females ranges from 12.0 to 15.5 gm/dl. In our study least value of Hb observed is 7.5 gm/dl and highest of 14.1 gm/dl mean value of 11.56 gm/dl which less than the normal limit. In a study by Deo et al Hb levels ranged from 7.20 to 13.40 (mean: 10.97 ± 1.32)gm%; anemia (Hb<12gm%) was present in 99 (73.4%) [6], which is almost comparable with our study. Normal range of S. ferritin levels in adult females is 10-120 ng/ml. In our study least value of S. ferritin observed is 3.9 ng/ml and highest of 79.3ng/ml (range 3.9 to 79.3ng/ml), mean of 30.21 ng/ml which is towards the lower limit, 8 patients had very low ferritin levels (less than 10ng/ml) all of them had TE. Hairs are rapidly proliferating organ with much requirement of blood supply. There is strong relationship between nutrional deficiency and hair loss. As compared to previous studies iron deficiency can be a certain factor of developing or worsening FPHL especially in premenopausal female patients [13]. In a study by Rasheed et al full detail not needed which is again not significant like in our study [14]. In another study Shetty et al mean S.ferritin was 19.3 ± 4.7 in TE, 32.8 ± 19.6 in FPHL [15], which is almost comparable with our study.
Normal range of TSH in adult females is 0.5-5.0 mIU/ml. In our study least value of TSH observed was 0.49 mIU/ml and highest of 7.13 mIU/ml, 5 patients had hypothyroidism all of them belong to TE. In a study by Deo et al Thyroid disorders presenting23(17%) patients, including nine (6.7%) newly diagnosed ones, too, were not of statistical significance [6]. Diffuse hair loss is sometimes may be the early clinical features of thyroid disorders only. The thyroid hormone is most essential for the development and maintenance of the hair follicle.In androgenetic alopecia the interactions between thyroid hormones and androgens may contribute to the development of alopecia. In females, sometimes hypophyseal hypothyroidism may play a role in androgenetic alopecia [16].
In our study in TE cases most common dermoscopic findings were Yellow Dots in 83% patients, Thinning of hairs in 58.1%, Peripilar sign in 25.8% and Vellus Hairs in 12.9%, In a study by Shetty V et al [15]. Thin hair (81.8%), yellow dots (54.5%), peripilar erythema (18.2%) where in most common trichoscopic finding in TE cases was thinning of hairs in contrast to our study where yellow dots was the most common finding. In Telogen effluvium usually there is no variation in HDD. The yellow dot as observed by dermoscopy indicates dilation of the follicular infundibulum.
In FPHL, the findings were HDD > 20% in 8 (88.8%), Yellow Dots in 8 (88.8%) and Thinning of hairs in 8 (88%), in a study by Shetty V et al HDD>20% (66.7%), thin hair (66.7%), yellow dots (55.6%) which were less when compared to our study [15]. In FPHL there is variability in thickness of hairs in frontal region compared occipital almost in all cases. The HDD variability it indicates the process of miniaturisation. The inflammatory process around the follicular area seen as slight desquamated scales or brownish halo which is called as peripilar sign observed in FPHL. Some times the seborrheic dermatitis would be associated with FPHL manifesting as yellowish scales on dermascopy. The diagnostic criteria proposed based on dermoscopic findings for FPHL [17–19] as follows: the major criteria are more than four yellow dots in four fields in the frontal area, decreased average shaft thickness in the frontal areacompared to the occipital scalp and more than 10% of thin hairs (thinner than 0.03 mm) in the frontal area and the minor criteria are perifollicular discoloration, increased single-hair pilosebaceous units in the frontal area compared to the occipital area, and increased vellus hairs in the frontal area compared to the occiput. Two major criteria and/or one major and one minor criterion are essential diagnose the FPHL. With these above findings the differences between female androgenetic alopecia and telogen effluvium can be made easily and specifically.
CONCLUSION
Any chronic hair loss female patent should be thoroughly evaluated for all possible causes include iron level, thyroid disorders and other laboratory test. The role of Dermascopy in evaluation of hair loss is most essential. The study has its limitation of small size and a thorough complete laboratory work up not done as our centre is poor in resource. With the usage of simple dermascopy we may able to differentiate various types of hair loss in females and also useful in follow up of the patients. In TE cases most common dermoscopic findings were Yellow Dots, In FPHL most common and specific finding was HDD > 20%. The need for the histopathological work up can be minimised with usage of dermascopic examination but however in some cases biopsy is must.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Werner B, Mulinari-Brenner F. Clinical and histological challenge in the diagnosis of diffuse alopecia:female androgenetic alopecia, telogen effluvium and alopecia areata – Part II. An Bras Dermatol. 2012;87:884-90.
2. Ruiz-Tagle SA, Figueira MM, Vial V, Espinoza-Benavides L, Miteva M. Micronutrients in hair loss. Our Dermatol Online. 2018;9:320-8.
3. Thokchom N, Verma K, Hafi BNA, Kshetrimayum S, Hmar V, Kongbam L, et al. A study on the association between thyroid dysfunction and various dermatoses in northeastern India. Our Dermatol Online. 2022;13:143-7.
4. Bhat YJ, Saqib N, Latif I, Hassan I. Female pattern hair loss—An update. Indian Dermatol Online J. 2020;4:493-501.
5. Hard S:Non-anemic iron deficiency as an etiologic factor in diffuse loss of hair of the scalp in women. ActaDermVenereol. 1963;43:562–9.
6. Deo K, Sharma Y K, Wadhokar M, Tyagi N. Clinicoepidemiological observational study of acquired alopecias in females correlating with anemia and thyroid function. Dermatol ResPrac.2016;2016:6279108.
7. Jain N, Doshi B, Khopkar U. Trichoscopy in alopecias:Diagnosis simpli?ed. Int J Trichol. 2013;5:170-8.
8. Agarwal S, Mendiratta V, Yadav P, Chander R. Diffuse hair loss in females. Indian Dermatol Online J. 2019;10:73-4.
9. Ramatulasi S, Annapurna Malladi M, Sri Satya R, Srujan Kumar K, Acharya A. Clinico -laboratory and trichoscopic evaluation of various patterns of female baldness –A prospective case control study. Indian J Clin Exp Dermatol. 2019;5:195-201.
10. Ummiti A, Ps P, Chandravathi PL, Kumar CS. Correlation of trichoscopic ?ndings in androgenic alopecia and the disease severity. Int J Trichol. 2019;11:118–40.
11. Shilpashree P, Ravikiran. An epidemiological study of female pattern hair loss at a referral centre in South India Indian. J Clin Exp Dermatol. 2016;2:106–10.
12. Tandon S, Arora P, Gautam RK, Bhardwaj M, Garga U, Sharma N. Correlation between clinical features, biochemical parameters, and histopathological findings in women with patterned baldness:Study from North India. J Cutan Aesthet Surg. 2019;12:42-8.
13. Park SY, Na SY, Kim JH, Cho S, Lee JH. Iron plays a certain role in patterned hair loss. J Korean Med Sci. 2013;28:934-8.
14. Rasheed H, Mahgoub D, Hegazy R, El-komy M, Hay RA, Hamid MA, et al. Serum ferritin and vitamin D in female hair loss:do they play a role?. Skin Pharmacol Physiol. 2013;26:101–7.
15. Shetty V, Eram H, Goel S, Babu AM. Dermoscopic study of hair loss in females and its correlation with serum ferritin levels. Journal of Pakistan Association of Dermatologists. 2019;29:322-7.
16. Vincent M, Yogiraj K. A Descriptive study of alopecia patterns and their relation to thyroid dysfunction. Int J Trichology. 2013;5:57-60.
17. Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Dermoscopy in female androgenic alopecia:method standardization and diagnostic criteria. Int J Trichology. 2009;1:123-30.
18. Chiramel MJ, Sharma VK, Khandpur S, Sreenivas V. Relevance of trichoscopy in the differential diagnosis of alopecia:A cross-sectional study from North India. Indian J Dermatol Venereol Leprol. 2016;82:651-8.
19. Nagar R, Dhudshia R. Utility of trichoscopy to diagnose early female pattern hair loss in resource-poor setting:A cross-sectional study. Indian J Dermatol Venereol Leprol. 2019;85:681.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|



Comments are closed.