A long-standing scar on the abdomen of healthy woman
Mohammed Shanshal 1, Nihull Jakharia-shah2
1, Nihull Jakharia-shah2
1Consultant dermatologist, Basildon University Hospital, UK, 2ST3 Speciality Trainee, Basildon University Hospital, UK
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
A 42-year-old woman presented with scar-like hyperpigmentation on the lower right abdominal quadrant. The lesion had been asymptomatic and had been present since early childhood following a varicella infection at the age of six. The patient initially believed the lesion to be a chickenpox scar and was reassured by her GP. The patient’s medical history included depression and fibromyalgia.
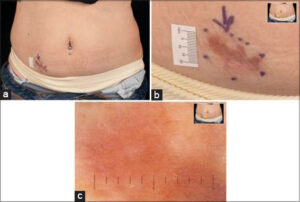
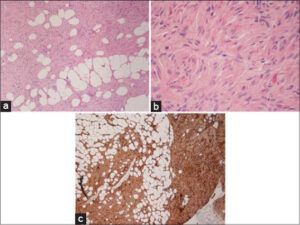
On examination, a scar-like atrophic hyperpigmented patch was detected (Figs. 1a and 1b). Dermoscopy showed a brownish, structureless background with no pigment network (Fig. 1c). On palpation, a 3 x 1.5 cm deeply seated nodule was detected. A fast-tracked ultrasound was ordered and revealed a well-defined hyperechoic solid lesion with no vascularity (Fig. 2). Due to clinical suspicion, a deep incisional biopsy was performed (Fig. 3a – 3c).
QUESTION 1: WHAT IS THE MOST LIKELY DIAGNOSIS?
-
A. Cellular dermatofibroma
-
B. Piloleiomyoma
-
C. Dermatofibrosarcoma protuberans
-
D. Nodular fasciitis
-
E. Plexiform neurofibroma
A- Cellular Dermatofibroma – Incorrect
A histologic variant of dermatofibroma, which shares the same clinical features as classical dermatofibroma. It presents as a firm papule, plaque, or nodule. Histologically, it is characterized by highly cellular with a dense aggregation of fibrohistiocytic cells distributed among coarse collagen bundles with a tendency for subcutaneous extension. As opposed to diffuse staining in DFSP, CD34 is usually positive at the periphery of the lesion [1].
B- Piloleiomyoma – Incorrect
Piloleiomyoma is a soft tissue tumor that arises from smooth muscles. It presents as a solitary or multiple firms and usually painful papules or nodules. It can develop sporadically or as part of Reed syndrome [2]. Histologically, it is characterized by interweaving bundles of smooth cells admixed with collagen bundles.
C- Dermatofibrosarcoma Protuberans – Correct
DFSP is a rare and locally aggressive soft tissue cutaneous sarcoma. Typically, DFSP presents as a symptomatic, slowly enlarging indurated plaque or, less commonly, as a firm cutaneous nodule on the trunk and proximal extremities. Histologically, it shows spindle cells arranged in a storiform pattern.
D- Nodular Fasciitis – Incorrect
Pseudosarcomatous neoplasm presents as a rapidly growing subcutaneous nodule. Histologically, it is characterized by a highly cellular circumscribed mass composed of spindle cells in fibromyxoid stroma situated deeply in subcutaneous tissue, fascia, and/or muscles with increased mitotic activity [3].
E- Plexiform Neurofibroma – Incorrect
It occurs as a part of neurofibromatosis type 1 and presents as small, soft plaques or nodules which may have palpable cords of thickened nerve fibers (bag of worms). Histologically, it is composed of spindle cell bundles with wavy nuclei in myxoid and collagen stroma [4].
QUESTION 2: WHAT IS THE MOST COMMON GENETIC ABNORMALITY ASSOCIATED WITH THIS LESION
A. MYH9-USP6
B. COL1A1-PDGFB
C. FIP1L1–PDGFRA
D. TMPRSS2:ERG
E. BCR-ABL
A- MYH9-USP6- Incorrect
This fusion gene is present in most of the nodular fasciitis cases.
B- COL1A1-PDGFB- Correct
DFSP shows a characteristic translocation between chromosomes 17 and 22 known as t (17; 22) (q22; q13), leading to the formation of COL1A1-PDGFB fusion genes. This results in an increase in the expression of PDGFB, which is the key factor in tumorigenesis in most of DFSP cases. This translocation can be used as a diagnostic tool in cases with atypical morphology.
C- FIP1L1–PDGFRA- Incorrect
This fusion protein is found in some cases of hypereosinophilic syndrome.
D- TMPRSS2:ERG- Incorrect
This fusion gene is associated with prostate cancer.
E- BCR-ABL- Incorrect
This fusion gene is associated with chronic myeloid leukemia.
QUESTION 3: WHICH MOLECULARLY TARGETED THERAPY IS USED FOR ADVANCED CASES OF THIS LESION
A. Vismodegib
B. Cemiplimab
C. Vorinostat
D. Ipilimumab
E. Imatinib
A- Vismodegib- Incorrect
Vismodegib is used for advanced basal cell carcinoma.
B- Cemiplimab- Incorrect
Cemiplimab is used in advanced cutaneous squamous cell carcinoma.
C- Vorinostat- Incorrect
Vorinostat is used to treat cutaneous T-cell lymphoma.
D- Ipilimumab- Incorrect
Ipilimumab is used for malignant melanoma.
E- Imatinib- Correct
Imatinib, a tyrosine kinase inhibitor, has been reported as a useful option for advanced unresectable, metastatic, and/or recurrent DFSP [5].
REFERENCES
1. Gaufin M, Michaelis T, Duffy K. Cellular dermatofibroma:clinicopathologic review of 218 cases of cellular dermatofibroma to determine the clinical recurrence rate. Dermatol Surg. 2019;45:1359-64.
2. Bhola PT, Gilpin C, Smith A, Graham GE. A retrospective review of 48 individuals, including 12 families, molecularly diagnosed with hereditary leiomyomatosis and renal cell cancer (HLRCC). Fam Cancer. 2018;17:615-20.
3. Al-Hayder S, Warnecke M, Hesselfeldt-Nielsen J. Nodular fasciitis of the face:a case report. Int J Surg Case Rep. 2019;61:207-9.
4. Khajavi M, Khoshsirat S, Ahangarnazari L, Majdinasab N. A brief report of plexiform neurofibroma. Curr Prob Cancer. 2018;42:256-60.
5. Navarrete-Dechent C, Mori S, Barker CA, Dickson MA, Nehal KS. Imatinib treatment for locally advanced or metastatic dermatofibrosarcoma protuberans:a systematic review. JAMA Dermatol. 2019;155:361-9.
Notes






Comments are closed.