A peculiar in situ case of cutaneous leukocytoclastic vasculitis induced by urinary infection
Ana Maria Abreu Velez 1, Bruce R. Smoller2, Michael S. Howard1
1, Bruce R. Smoller2, Michael S. Howard1
1Georgia Dermatopathology Associates, Atlanta, Georgia, USA, 2Department of Pathology and Laboratory Medicine, University of Rochester Medical Center and School of Medicine and Dentistry, Rochester, New York, USA
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Cutaneous leukocytoclastic vasculitis (LCV) is a disease thought to be related to the presence of immune complex deposition within small blood vessel walls. A 58-year-old female presented with purpuric papules on both legs, occurring concurrent with urinary symptoms. Histologically, an intraepidermal blister with luminal fragmented neutrophils was present. A dermal infiltrate was observed surrounding blood vessels; mild perivascular leukocytoclastic debris favored a diagnosis of LCV. Both direct immunofluorescence and immunohistochemistry staining also favored a diagnosis of LCV. Reactivity of the involved vessels was observed to multiple antibodies and complement, with overexpression of von Willebrand factor, CD15, and CD45. The ribosomal protein Phospho-S6 was also detected within the involved vessels, and on the blister roof and floor. The clinical triggering factor was a urinary infection that had progressed to urosepsis; the symptoms were effectively treated. We present an unusual case, where multiple immune reactions correlate with clinical and histologic changes characteristic of LCV.
Key words: Cutaneous leukocytoclastic vasculitis, Sialyl Lewis, CD45, Von willebrand factor
Abbreviations
Cutaneous leukocytoclastic vasculitis (CLCV), leukocytoclastic vasculitis (LCV), small vessel vasculitis (SVV), von Willebrand factor (VWF), Sialyl Lewis (sLeX), anti-neutrophil cytoplasmic antibody (ANCA), neutrophil extracellular traps (NETs), direct immunofluorescence (DIF), basement membrane zone (BMZ).
INTRODUCTION
Cutaneous leukocytoclastic vasculitis (LCV) is a small vessel vasculitis illustrated histopathologically by the presence of immune complex-mediated vasculitis of the dermal capillaries and venules on the skin [1]. The majority of cases are idiopathic; however, infections and medications are the most frequent triggers. Systemic diseases could also be the cause of LCV [1].
CASE REPORT
A 58-year-old female presented with acute onset of a skin rash that was present for one week; she also reported urinary symptoms, but no abdominal pain or headache. The skin rash began as a sudden eruption of pruritic, erythematous-to-violaceous, non-blanchable macules and papules involving the lower extremities (Fig. 1a). Fever was also present. There was no history of recent drug intake; her kidney function testing was normal. Hepatitis B and C, as well as HIV titers were negative. Leukocytosis was present. Her hemoglobin, extractable nuclear antibodies panel, anti-Complement C1Q antibodies, cryoglobulins, and Complement C3 and C4 levels were normal. There were no red blood cell fragments or hemolysis detected. Serum levels of IgG, IgA, and IgM were all within normal limits. Her C reactive protein was elevated, and urinalysis was abnormal, showing cloudy urine. Urinary culture and blood cultures were obtained, and both were positive for gram-negative rods. A diagnosis of sepsis secondary to urinary tract infection (UTI) (urosepsis) was established by isolating Proteus spp, and accordingly intravenous administration of Ceftriaxone was initiated.
The skin biopsy microscopic examination demonstrated a focal intraepidermal vesicle formation with neutrophils and other fragmented cellular debris in the vesicle lumina. An inflammatory infiltrate was present in and around the dermal blood vessels, including those within dermal papillae (Fig. 1b). Overall, there was a florid inflammatory process involving capillaries and small blood vessels within the superficial dermis. Fibrin deposition and fibrinoid necrosis were observed within blood vessel walls; neutrophil infiltration was present within the vessel walls, accompanied by fragmented neutrophil nuclei, extravasated erythrocytes, and some perivascular lymphocytes (Fig. 1c). A diagnosis of cutaneous leukocytoclastic vasculitis (LCV) was rendered. After five days of antibiotics and low dose corticosteroids, there was clinical improvement of the skin lesions.
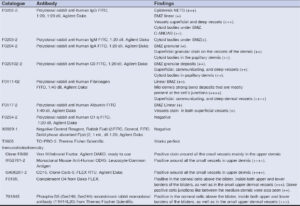
Further testing was performed using direct immunofluorescence (DIF), as well as immunohistochemical (IHC) studies. Please see Table 1 for the used antibodies. Our results are shown in Figure 1. In Figure 1b, we demonstrate the leukocytoclastic vasculitis (LCV). In Figure 1c, double IHC staining shows a subepidermal blister with staining around the blister demonstrates the presence of Ribosomal Protein Phospho-S6 in the top of the blister as well as on the blister floor and blood vessel walls. IHC also shows staining in the dermis using antibodies against Complement 4. In Figure 1d, the DIF stain is positive for FITC conjugated antibodies directed against human fibrinogen, were positive in the area of a dermal papilla, some weak basement membrane zone, and dermal vessels. Figure 1e shows double IHC staining using an antibody to von Willebrand Factor colocalizing with positive staining for CD45 antibody (Fig. 1f). IHC stain showing positive staining with CD51 stain around upper dermal vessels colocalizing with an antibody directed against CD15.
DISCUSSION
Leukocytoclastic vasculitis (LCV) and cutaneous leukocytoclastic vasculitis (CLCV) (limited to the skin) are sometimes associated with other immune reactions, and/or medications, collagen-vascular diseases, infections, paraproteinemias, vaccines, biologic treatments, and neoplasias. About 50% of cases remain idiopathic [2]. LCV is a histopathologic manifestation of a common form of small vessel vasculitis (SVV) that can involve the skin and internal organs [2,3]. LCV can present as part of a systemic disease, most frequently involving ANCA-associated vasculitides, connective tissue diseases, cryoglobulinemia’s, IgA vasculitis (formerly known as Henoch-Schönlein purpura) and hypocomplementemic urticarial vasculitis [4]. When LCV is suspected, an extensive work-up is usually necessary to determine whether the process is skin-limited, or a manifestation of systemic vasculitis or disease.
We present a case of leukocytoclastic vasculitis, manifested initially by cutaneous lesions. The case was linked to a urinary infection that became systemic; this phenomenon has been previously described [5].
In addition to the histological features clearly showing an LCV, the complementary DIF and IIF studies demonstrated immune complexes in the dermal blood vessels; we observed complement, fibrinogen, and immunoglobulin IgA positivity. Additional and unusual findings in our case included strong positivity for von Willebrand Factor (VWF), CD15, CD45, complement C4 and Phospho-S6 Ribosomal Protein (Ser240/244).
In our case, Ribosomal Protein Phospho-S6 (Ser240, Ser244) implicates an immune process that may represent an intermediate stage between the cutaneous and the systemic form of LCV. Notably, the deposition of complement and immunoglobulins, as well as fibrinogen at the dermal epidermal junction has been reported by others [6]. Multiple studies focusing on the DIF findings of LCV have not reported the complex local immune responses in skin biopsies that we present here [7,8].
The presence of VWF has not been studied properly in LCV. In our case, the anti-VWF antibody was strongly positive and colocalized with CD45 and CD15 and with Ribosomal Protein Phospho-S6 (Ser240/244). Recent studies highlight the involvement of VWF and its regulator, ADAMTS13, in mechanisms that underline a possible bridge between vascular inflammation and immunothrombosis [9]. VWF and ADAMTS13 seem to play roles in leukocyte rolling, adhesion, extravasation, vascular permeability, ischemia/reperfusion injury, complement activation, and NETosis [8].
In our case, we used antibodies directed against CD15 [Sialyl LewisX(sLeX), a stage-specific embryonic antigen] what has been shown to be important in leukocyte tethering and rolling [10]. In our case, it appears that CD15 possibly contributed to leukocyte tethering to the vascular endothelium. The tethering role may explain how CD15 was expressed in a co-localized manner with the observed CD45 positivity [10].
CONCLUSION
Leukocytoclastic vasculitis is a process that can be triggered by many factors, and the characteristics of the biopsy-correlating immune and inflammatory response must thus be studied accordingly.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki. The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published, and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Baigrie D, Goyal A, Crane JS. Leukocytoclastic Vasculitis. 2023 Aug 8. In:StatPearls [Internet]. Treasure Island (FL):StatPearls Publishing;2023 Jan–. PMID:29489227.
2. Fraticelli P, Benfaremo D, Gabrielli A. Diagnosis and management of leukocytoclastic vasculitis. Intern Emerg Med. 2021;16:831-41.
3. Chango Azanza JJ, Calle Sarmiento PM, Lopetegui Lia N, Alexander SA, Modi V. Leukocytoclastic vasculitis:an early skin biopsy makes a difference. Cureus. 2020;12:e7912.
4. Bhesania S, Raol K, Medina C, Ilyas S, Bhesania J, Barmanwalla A. Leukocytoclastic vasculitis:depiction of the diagnostic dilemma. Cureus. 2021;13:e17462.
5. Abdelnabi M, Cavazos A, mitral N, tarbox M. Leucocytoclastic vasculitis secondary to urinary tract infection caused by extended-spectrum b-lactamase producing Klebsiella pneumonia. BMJ Case Rep. 2023;16:e2555395.
6. Magro CM, Mo JH, Kerns MJ. Leukocytoclastic vasculitis in association with linear epidermal basement membrane zone immunoglobulin deposition:Linear vasculitis. Clin Dermatol. 2022;40:639-50.
7. Lath K, Chatterjee D, Saikia UN, Saikia B, Minz R, De D, et al. Role of direct immunofluorescence in cutaneous small-vessel vasculitis:experience from a tertiary center. Am J Dermatopathol. 2018;40:661-6.
8. Takatu CM, Heringer APR, Aoki V, Valente NYS, de Faria Sanchez PC, de Carvalho JF, et al. Clinicopathologic correlation of 282 leukocytoclastic vasculitis cases in a tertiary hospital:a focus on direct immunofluorescence findings at the blood vessel wall. Immunol Res. 2017;65:395-401.
9. Gragnano F, Sperlongano S, Golia E, Natale F, Bianchi R, Crisci M, et al. The role of von willebrand factor in vascular inflammation:from pathogenesis to targeted therapy. Mediators Inflamm. 2017;2017:5620314.
10. Zhang Y, Ohkuri T, Wakita D, Narita Y, Chamoto K, Kitamura H, et al. Sialyl lewisx antigen-expressing human CD4+T and CD8+T cells as initial immune responders in memory phenotype subsets. J Leukoc Biol. 2008;84:730-5.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-7692-4133 http://orcid.org/0000-0002-7692-4133 http://orcid.org/0000-0002-7301-8274 http://orcid.org/0000-0002-7301-8274 http://orcid.org/0000-0003-0430-6093 http://orcid.org/0000-0003-0430-6093 |





Comments are closed.