Dermoscopy of vulvar pigmented lesions: A series of 59 cases
Kalmi Noura , Hanane Baybay, Choukri Souad, Zakia Douhi, Sara Elloudi, Soughi Meryem, Fatima Zahra Mernissi
, Hanane Baybay, Choukri Souad, Zakia Douhi, Sara Elloudi, Soughi Meryem, Fatima Zahra Mernissi
Department of Dermatology, University Hospital Hassan II, Fes, Morocco
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Due to the lack of a large series of benign and malignant vulvar lesions, the features of dermoscopy are not well established.
Objective: The aim of our study was to describe the epidemiological profile and the clinical and dermoscopic characteristics that may indicate malignancy or benignity in vulvar hyperpigmentation.
Materials and Methods: From June 2020 to June 2023, we conducted a retrospective, prospective study involving 42 patients with 59 pigmented lesions.
Results: The parallel, homogeneous, and globular patterns were observed in benign lesions (nevi, lentigo, melanosis). The cerebriform pattern was observed in seborrheic keratosis and Bowen’s disease (BD). In cases of BD, we also observed white, structureless areas, glomerular vessels, a homogeneous brownish-gray area, and brown dots.
Conclusion: Good clinico-dermoscopic correlation should guide the diagnosis and management of pigmented vulvar lesions. Dermoscopy may be helpful in distinguishing between a benign lesion and a malignant lesion, yet in cases of doubt, a biopsy may be necessary.
Key words: Dermoscopy, Vulvar, Pattern, Pigmented
INTRODUCTION
Pigmented vulvar lesions include a wide range of conditions that may be either of melanocytic origin by hyperplasia (nevus, melanoma) or epithelial hyperpigmentation without significant melanocytic hyperplasia (post-inflammatory pigmentation, vulval melanosis, and lentigo), or of non-melanocytic origin (seborrheic keratosis, vulval intraepithelial neoplasia (VIN), condyloma, angiokeratoma). Vulvar melanoma is a rare gynecological disease that accounts for 5% of all neoplasms of the vulva [1] and 3–7% of all women’s melanomas [2]. Early melanoma may share some clinical and dermoscopic features with benign lesions and may, therefore, be a source of anxiety for both the patient and the dermatologist [3]. Diagnosing melanoma based on clinical criteria alone is often unreliable, and histological examination is considered necessary. However, dermoscopy is a non-invasive tool that helps doctors differentiate melanoma from other pigmented and non-pigmented skin lesions. It provides numerous clues about the structure of the skin in the epidermis, the dermoepidermal junction, and the dermis. For the detection of lesion borders, numerous methods have been developed [4]. To our knowledge, there have been few studies on the dermoscopic features of pigmented vulvar lesions. The aim of our study was to describe the epidemiological profile and the clinical and dermatoscopic characteristics of vulvar hyperpigmentation.
MATERIALS AND METHODS
This retrospective, prospective study evaluated the clinical and dermoscopic assessment of pigmented vulvar lesions. However, histological examination was performed in cases of suspected malignancy. The sample of patients was collected on consultation at the Dermatology Department of the HASSAN II University Hospital of Fez in Morrocco during the period from June 2020 to June 2023. The pictures analyzed were taken using a digital dermoscopy system (Dermatoscope DermLite DL4). The latter was packaged in disposable food packaging to avoid microbiological contamination. The dermoscopic images were taken by the same dermatologist to avoid diversification during the procedure, and the dermoscopic images were evaluated by two different dermatologists. The selection of dermoscopic patterns was based on the results of the literature [4,5].
RESULTS
A total of two hundred women were examined, among whom 42 presented with 59 pigmented lesions. The age range was 4–79 years (median: 42). Histological examination was performed in twelve patients, with four cases of suspected melanoma, four cases of Bowen’s disease (BD), and four cases of diagnostic uncertainty between condyloma and seborrheic keratosis (SK). We collected 16 cases of melanosis, 13 of seborrheic keratosis, 10 of condylomas, 9 of nevi, 7 of lentigo, and 4 of Bowen’s disease.
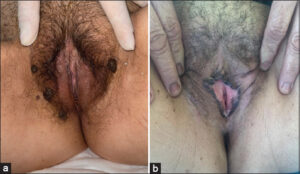
The clinical appearance of the lesion (Table 1) was raised in 38 cases and the macular in 21 cases (Figs. 1a and 1b). It was multifocal in 35 cases, unifocal in 24 cases, and melanosis was in most cases multifocal, contrary to lentigo and nevi. Lesions were preferentially located on the labia majora (37 cases vs. 16 cases on the labia minora, 4 cases on the posterior fourchette, and 2 cases on the clitoris). 35 lesions were larger than 6 mm, and 24 lesions were smaller than 6 mm.
Dermoscopic analysis of our pigmented lesion series was performed.
For melanosis, the parallel pattern was observed in 12 cases, and the homogeneous pattern in 4 cases. As for lentigo, the parallel pattern was observed in 5 cases (Fig. 2a), and the homogeneous pattern in 1 case. For nevi, the globular pattern was observed in 3 cases (Fig. 2b), the homogenous, brown pattern in 3 cases (Fig.2c), and the bicomponent pattern with a peripheral reticular pattern and central homogeneous pattern in 1 case (Fig. 2d).
Meanwhile, we suspected melanoma in 4 cases. The first patient presented a recent appearance of a pigmented clitoral macule with a hyphal pattern and irregular dots on dermoscopy (Fig. 2e). Histology was suggestive of lentigo. The second patient had a history of acral melanoma and presented a pigmented vulvar macule. Dermoscopy revealed a multicomponent pattern, irregular dots and globules, and a bluish-white veil (Fig. 2f). Histology confirmed the diagnosis of melanosis. The third patient had an uncertain date of onset of a small, blue, vulvar papule with a homogeneous blue pattern on dermoscopy, which was histologically confirmed as a blue nevus. The last patient, a teenager, presented with an increase in the size and thickness of her congenital vulvar lesion. Dermoscopy revealed a blackish-blue background, a bluish-white veil, and chrysalises, confirming the diagnosis of a deep-penetrating blue nevus by histology. As for the dermoscopy of seborrheic keratosis, we noted well-defined lesions with a papillomatous pattern in 8 cases, comedo-like openings in 7 cases (Fig. 2g), fingerprint-like structures in 2 cases, a vascular pattern in 2 cases, and milia-like cysts in 1 case. (Fig. 2h) In seborrheic keratosis-like condylomas, we observed a papillomatous pattern in 9 cases, a finger-like pattern in 1 case, a mixed pattern in 1 case (Fig. 2i), a vascular pattern in 9 cases, and a knob-like pattern in 7 cases (Fig. 2j). For patients with Bowen’s disease, the cerebriform pattern was observed in 2 cases, white, structureless areas in 3 cases, erosions in 2 cases, glomerular vessels in 3 cases, polymorphic vessels in 1 case, a homogeneous, brownish-gray area in 4 cases, and brown dots in 1 case. (Figs. 2k and 2l) (Table 1).
DISCUSSION
Dermoscopy, as a non-invasive technique, has become a fundamental element in the assessment of skin and mucosal lesions, whether pigmented or not, while improving diagnostic accuracy. However, the examination and assessment of mucosal lesions may be difficult to perform. First of all, especially in female patients, the lesion may be in a place that is difficult to examine and the patient may feel embarrassed. Secondly, the stretching of the mucosa during the examination could affect the dermoscopic findings. Thirdly, the contact probes should be protected in order to avoid the risk of infection [6,7].
Pigmented lesions represent a wide range of conditions, from benign to malignant, infectious to post-inflammatory [8]. Dermoscopically, they show various patterns with somewhat confusing terminology, including the homogeneous or structureless pattern, the globular pattern, the parallel pattern with its variants, the ring-like pattern with its variants, and the fish scales, and hyphal patterns, which are probably due to external technical issues related to lesion examination with a dermoscope or photographing techniques [9]. It is also possible to identify specific dermoscopic features of other etiologies, such as comedo-like openings and milia-like cysts.
Melanosis, also known as vulvar lentiginosis and melanotic macule, the most common pigmented vulvar lesion in reproductive women [10], typically presents as pigmented macules, often multifocal, and is more common in individuals with darker skin phototypes [11]. Vulvar melanosis is more commonly reported in perimenopausal women [12]. Histologically, melanosis is due to an increase in pigmentation restricted to the basal keratinocytes and melanocytes [13,14]. Dermoscopically, vulvar melanosis presents with either a homogeneous or heterogeneous pattern of brown or black shades without red, gray or blue colors and generally without the typical melanocytic dermoscopic structures [12]. Various patterns have been described, including the structureless, parallel, reticular-like, cobblestone-like, ring-like, and globular patterns. Our results are consistent with the literature and show a predominance of the parallel and homogeneous patterns [5,12].
Vulvar nevi, on the other hand, represent 2.3% of melanocytic nevi cases [15]. They often appear in childhood with dermoscopic patterns similar to their cutaneous counterparts [11]. The most common patterns are the globular and homogeneous [5,11]. Common nevi generally have a single or bicomponent pattern, rarely a multicomponent pattern, in contrast to atypical melanocytic nevi of the genital type, which may be observed in reproductive age and which have a multicomponent pattern and may mimic malignant melanoma. The whitish-blue veil and irregular dots may also be seen [4]. Our results are consistent with the literature, with a predominance of globular and homogeneous patterns [4,11,12].
Meanwhile, in the presence of a multicomponent pattern, melanoma should be considered, especially if associated with heterochromia, white, gray, blue, or red colors, asymmetrically distributed irregular dots and globules, irregular and atypical vessels have also been described [16,17]. Fortunately, we have not found any cases of melanoma of the vulva in our series.
Bowen’s disease presents dermoscopically with bluish-grey dots and globules in a linear arrangement and glomerular or dotted vessels. The cerebriform pattern and hypo- or hyperpigmented structureless areas have also been described [18,19]. Our results are also consistent with case reports in the literature, with a predominance of homogeneous brown areas and glomerular vascularization, although the cerebriform pattern was more common in our cases [18].
Seborrheic keratoses, unusual benign lesions of the genitalia, present with a papillomatous pattern on dermoscopy, which may overlap with Bowen’s disease and pigmented vulvar intraepithelial neoplasia, yet there are milia-like cysts and comedo-like openings [5,20], which is consistent with the literature regarding the predominance of the papillomatous pattern and the presence of comedo-like opening [4].
Condylomas acuminata present with different patterns, including the cerebriform, depending on the age of the lesions, yet with less pigmented than seborrheic keratosis. They generally lack milia-like cysts and comedo-like openings and have prominent vascular patterns and vessels surrounded by a white halo [20]. Thus, the cerebriform pattern may be seen in benign lesions such as SK, yet we have milia-like cysts, rarely comedo-like openings as a result of maceration [20]. It may also be seen in malignant lesions such as Bowen’s disease and vulvar intraepithelial neoplasia, yet we have bluish-gray dots in a linear arrangement and glomerular vascularization. [18,20].
CONCLUSION
Despite the predominance of benign pathologies, we must always be wary of atypical cases, such as those of our patients, who presented with a compound pattern and whose biopsy excluded melanoma. We should also be wary of any cerebriform pattern and fear Bowen’s disease and VIN in the absence of milia-like cells and comedo-like openings in the presence of glomerular vessels, peripheral brown dots and globules in a linear arrangement, and white or brown structureless areas.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Falcicchio G, Vinci L, Cicinelli E, Loizzi V, Arezzo F, Silvestris E, et al. Vulvar malignant melanoma:A narrative review. Cancers (Basel). 2022;1421:5217.
2. Dunton CJ, Berd D. Vulvar melanoma, biologically different from other cutaneous melanomas. Lancet. 1999;3549195:2013-4.
3. Lenane P, Keane CO, Connell BO, Loughlin SO, Powell FC. Genital melanotic macules:clinical, histologic, immunohistochemical, and ultrastructural features. J Am Acad Dermatol. 2000;424:640-4.
4. Cengiz FP, Emiroglu N, Wellenhof RH. Dermoscopic and clinical features of pigmented skin lesions of the genital area. An Bras Dermatol. 2015;902:178-83.
5. Ronger-Savle S, Julien V, Duru G, Raudrant D, Dalle S, Thomas L. Features of pigmented vulval lesions on dermoscopy. Br J Dermatol. 2011;1641:54-61.
6. Hofmann-Wellenhof R. Special criteria for special locations 2:Scalp, mucosal, and milk line. Dermatol Clin. 2013;314:625-ix.
7. Cinotti E, Campoli M, Pataia G, Ouerdane Y, Thuret G, Gain P, et al. How transparent film applied on dermatologic imaging devices in order to prevent infections affects image quality?Skin Res Technol. 2019;252:229-33.
8. Edwards L. Pigmented vulvar lesions. Dermatol Ther. 2010;235:449-57.
9. Lin J, Koga H, Takata M, Saida T. Dermoscopy of pigmented lesions on mucocutaneous junction and mucous membrane. Br J Dermatol. 2009;1616:1255-61.
10. Rock B, Hood AF, Rock JA. Prospective study of vulvar nevi. J Am Acad Dermatol. 1990;221:104-6.
11. Kamat D, Vinay K. Dermatoscopy of nonvenereal genital dermatoses:A brief review. Indian J Sex Transm Dis AIDS. 2019;401:13-9.
12. De Giorgi V, Gori A, Salvati L, ScarfìF, Maida P, Trane L,et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;15611:1185-91.
13. Venkatesan A. Pigmented lesions of the vulva. Dermatol Clin. 2010;284:795-805.
14. Heller DS. Pigmented vulvar lesions:A pathology review of lesions that are not melanoma. J Low Genit Tract Dis. 2013;173:320-5.
15. Mannone F, De Giorgi V, Cattaneo A, Massi D, De Magnis A, Carli P. Dermoscopic features of mucosal melanosis. Dermatol Surg. 2004;308:1118-23.
16. De Pascalis A, Perrot JL, Tognetti L, Rubegni P, Cinotti E. Review of dermoscopy and reflectance confocal microscopy features of the mucosal melanoma. Diagnostics (Basel). 2021;111:91.
17. Vaccari S, Barisani A, Salvini C, Pirola S, Preti EP, Pennacchioli E, et al. Thin vulvar melanoma:A challenging diagnosis. Dermoscopic features of a case series. Clin Exp Dermatol. 2020;452:187-93.
18. Narahira A, Yanagi T, Kitamura S, Hata H, Shimizu H. Dermoscopic features of genital pigmented Bowen’s disease:Report of a case and review of the published work. J Dermatol. 2019;4610:e390-1.
19. Maatouk I, Apalla Z, Errichetti E, Lallas A. Dermoscopy for venereologists:An update on patterns of tumors, inflammatory and infectious diseases of the genitalia, and tips for differential diagnosis. Int J Dermatol. 2021;6010:1211-8.
20. Seong SH, Jung JH, Kwon DI, Lee KH, Park JB, Baek JW, et al. Dermoscopic findings of genital keratotic lesions:Bowenoid papulosis, seborrheic keratosis, and condyloma acuminatum. Photodiagnosis Photodyn Ther. 2021;36:102448.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-3455-3810 http://orcid.org/0000-0003-3455-3810 http://orcid.org/0000-0002-5942-441X http://orcid.org/0000-0002-5942-441X |






Comments are closed.