Serum lipid profile pattern in lichen planus: A cross-sectional study in northeast India
Deepa Yumnam 1, Bhavya Valsalan2, Mrudula Sudharmman2, Thangjam Bijayanti Devi2, Thokchom Nandakishore2, Nischith V Kadam2
1, Bhavya Valsalan2, Mrudula Sudharmman2, Thangjam Bijayanti Devi2, Thokchom Nandakishore2, Nischith V Kadam2
1Manipur Health Services, Manipur, India, 2Department of Dermatology, Regional Institute of Medical Sciences, Imphal, Manipur, India
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Lichen planus (LP) is a chronic idiopathic T cell-mediated papulosquamous disorder clinically characterized by firm, shiny, polygonal, pruritic, 1–3 mm, flat-topped, erythematous to violaceous papules and plaques. Oral LP has a characteristic whitish streak in a reticular pattern (Wickham striae). Recent scientific literature has emphasized the strong association between chronic inflammation in LP and cardiovascular disease. This study was undertaken to study if dyslipidemia is associated with LP.
Materials and Methods: After informed consent, thirty-eight willing patients aged between 10 and 80 years of age with clinical presentations suggestive of lichen planus who had attended the Dermatology OPD of RIMS in Manipur, India, were included in the study. Histopathology was done to confirm doubtful cases. Detailed history, examination, and investigations were conducted for all patients. Dermoscopic examination was done to aid in clinical diagnosis.
Results: Females outnumbered males (m/f: 1:1.7). A majority of the patients (84.2%) had a duration of disease above six months. Dyslipidemia was found in 50% of the patients (n = 19). The mean levels of HDL, TC, LDL, TG, and VLDL were 47 ± 8.89 mg%, 166.11 ± 34.93 mg%, 107.95 ± 26.17 mg%, 152.95 ± 63.46 mg%, and 19.55 ± 5.94 mg%, respectively. The single most common lipid abnormality found was deranged HDL (13.2%), followed by abnormal TC (10.5%), TG (7.9%), and deranged VLDL (2.6%). 15.8% of the patients had multiple lipid abnormalities. The 40–49 year (31.5%) group was the most commonly associated with dyslipidemia, which was statistically significant. Dyslipidemia was the most commonly seen in the reticular type (42.1%) of LP, although not statistically significant. No statistically significant correlation was found between dyslipidemia and clinical types nor with the symptoms of LP.
Conclusions: Dyslipidemia was present in half of the patients, and the most common single abnormality was low HDL. A majority had multiple lipid abnormalities. A statistically significant portion of the patients in their 40s had dyslipidemia. Statistically significant abnormal TG and LDL were observed in age groups 40–49 years and 60–79 years, respectively. Hence, all patients with LP should undergo lipid profile testing for early detection and primary prevention of metabolic syndrome and cardiovascular complications.
Key words: Lichen planus, Inflammation, Dyslipidemia, Cardiovascular morbidity
INTRODUCTION
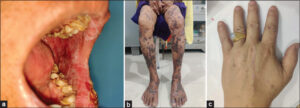
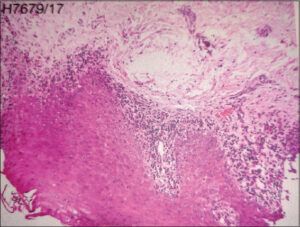
LP represents a CD8+ T cell-mediated autoimmune disease in which CD8+ T cells are recruited into the skin leading to interface dermatitis [1]. The activated lymphocytes release mediators such as IL-2, IL-4, IL-10, IFN –γ, TNF- α, and TGF-β and trigger apoptosis [2]. Clinically, it is characterized by pruritic, purplish, plane-topped papules and plaques with white streaks and koebnerization (Figs. 1a – 1c). On histopathology, epidermal changes such as hyperkeratosis, focal hypergranulosis, elongated rete ridges, basal cell degeneration, and focal dermoepidermal separation with band-like dense lymphocytic infiltrate in the papillary dermis are seen [1] (Fig. 2).
There is growing evidence of the association between LP and cardiovascular risk factors such as dyslipidemia. LP, being a chronic inflammatory condition, may explain this observation. High plasma IL-6 levels in oral LP were found to be associated with high total cholesterol (TC) and triglyceride (TG) and low high-density lipoprotein levels (HDL) [3,4].
Few studies have been done in this regard. If the association between LP and dyslipidemia is established, lipid level screening in patients with LP may be useful to detect individuals at risk and begin preventive treatment against cardiovascular disease.
This study, therefore, was undertaken to study if dyslipidemia is associated with LP.
MATERIALS AND METHODS
This was a cross-sectional, observational study conducted over a period of two years at the Dermatology OPD of RIMS in Imphal. Willing patients of both sexes and aged 10–80 years with lichen planus were included in the study after informed consent. Patient confidentiality was ensured.
Pregnant or lactating women and patients on treatment for LP in the past six months were excluded.
Consecutive sampling was employed. Both cases and controls were subjected to proper history taking, clinical examination, and laboratory tests. Dermoscopic examination was performed in doubtful cases. Fasting serum lipid profile was measured by enzymatic endpoint method.
Dyslipidemia was diagnosed based on the NCEP ATP III guidelines:
- Serum total cholesterol (TC) > 200 mg/dL.
- Serum triglycerides (TG) > 150 mg/dL.
- Serum low-density lipoprotein cholesterol (LDL-C) > 130 mg/dL.
- Serum high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL for males and < 50 mg/dL for females.
- If the patient was already receiving treatment for dyslipidemia.
Data was analyzed with SPSS, version 21.0. For inferential statistics, chi-squared/Fisher exact test was employed. A p value < 0.05 was considered statistically significant. Student t-test was employed to compare means.
Ethics Statement
Ethical approval was obtained from the institute ethics committee.
RESULTS
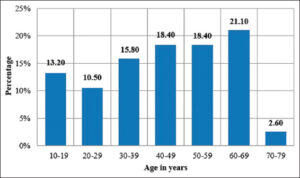
The age of the study population ranged from 11 to 72 years, with a majority in the age group of 60–69 years (21.1%) (Fig. 4). The mean and median age were 44.87 ± 17.51 years and 46.5 years, respectively. The age range for males and females were 11–72 years and 17–65 years, respectively.
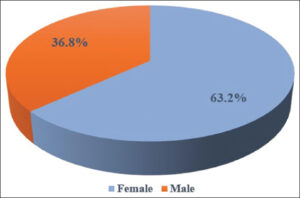
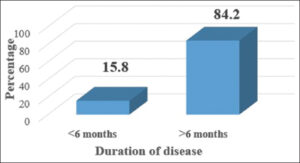
Females outnumbered males with a M: F ratio of 1:1.7 (Fig. 5). A majority had a duration of disease > 6 months (84.2%) (Fig. 6). The mean disease duration was 21.05 ± 28.81 months. The mean and median BMI were 24.23 ± 2.81 and 24.35, respectively.
Dyslipidemia was found in 50% of the cases (n = 19).
Table 1 shows the mean values and standard deviations of lipid profile parameters among the cases.
The mean levels of HDL, TC, LDL, TG, and VLDL were 47 mg/mL, 166.11 mg/mL, 107.95 mg/mL, 152.95 mg/mL, and 19.55 mg/mL, respectively.
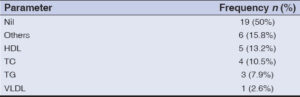
The most common single lipid abnormality was deranged HDL (13.2%), followed by abnormal TC (10.5%), TG (7.9%), and VLDL (2.6%). Multiple deranged lipid parameters were seen in 15.8, among which deranged TC with TG and deranged HDL with TG (5.3% each) was most commonly seen (Table 2).
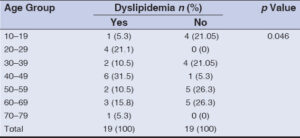
The most common age group associated with dyslipidemia was 40–49 years (31.5%) followed by 20–29 years (21.1%) (Table 3). This finding was statistically significant (p = 0.046).
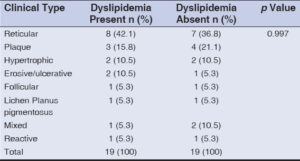
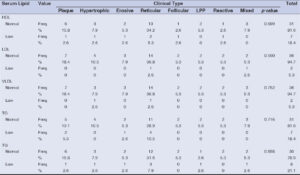
Dyslipidemia was most commonly seen in the reticular variant (42.1%), followed by the plaque (15.8%), hypertrophic (10.5%), and erosive (10.5%) types (Table 4). However, this finding was statistically insignificant.
Among symptomatic patients, abnormal TG, TC, HDL, LDL, and VLDL were seen in 21.6%, 18.9%, 18.9%, 5.4%, and 5.4% of the patients, respectively (Table 5). However, this finding was statistically insignificant.
Out of 18.4% of the patients with abnormal HDL, 5.3% had reticular LP, followed by 2.6% each for the plaque, hypertrophic, erosive, follicular, and reactive variants.
LDL was found to be raised in the reticular (2.6%) and mixed (2.6%) types whereas VLDL was found to be high in the hypertrophic (2.6%) and reticular types (2.6%).
Among patients with high TC, 10.5% had the reticular type, followed by 5.3% with the plaque type and 2.6% with the erosive type.
High TG was found in 7.9% with the reticular variant and 2.6% each with the plaque, hypertrophic, erosive, LPP, and mixed types.
However, the correlation between the clinical types of LP and dyslipidemia was found to be statistically insignificant (Table 4).
DISCUSSION
In this study, the age of the patients ranged from 11–72 years, with a majority of the patients in the age group of 60–69 years (21.1%). The mean and median age were 44.87 ± 17.51 years and 46.5 years, respectively, comparable to findings by Chalkoo et al. [5], Kar et al. [6], Arias Santiago et al. [7], Aniyan et al. [8], and Manasa et al. [9].
Females (63.2%) were more commonly affected than males (36.8%), similarly to other studies [10–13]. The male-to-female ratio was 1:1.7, similarly to findings by Chalkoo et al. [5] with a M: F ratio of 1:1.85. The mean and median BMI among the patients were 24.23 ± 2.81 and 24.35, respectively, comparably to findings by Hammam et al. [14] yet lower than in those by Manasa et al. [9] and Khan et al. [15].
The duration of the disease ranged from 1 month to 11 years. A majority of the patients had symptoms for more than six months (82.4%), similarly to other studies [7,12]. The mean age of disease duration was 21.05 ± 28.81 months, similarly to studies by Hashba et al. [12] (20.5 ± 26.1 months) and Arias Santiago et al. [7] (1.8 years in females, 1.6 years in males) yet longer than in studies by Ozkur et al. [16] (8.4 ± 8.3 months), which may be due to the neglect of symptoms by patients in our region.
Dyslipidemia was observed in 50% (n = 19) of the patients. This prevalence was higher than in findings by Agarwala et al. [17] with 35.9% of the patients having dyslipidemia, yet lower than in those by Azeez et al. [18] with 63.8% of the patients having dyslipidemia.
The mean for HDL was comparable to studies by Azeez et al. [18] and others [8,13,14]. It was higher than in studies by Kar et al. [6] and others [8,9], yet lower than in studies by Arias-Santiago et al. [7] and Özkur et al. [16].
The mean for TC was comparable to findings by Aniyan et al. [8] yet lower than those by Kar et al. [6].
The mean for LDL was comparable to findings by Aniyan et al. [8]. It was higher than findings by Manasa et al. [9] yet lower than those by Kar et al. [6] and others [7,10,11,16].
The mean for TG was comparable to findings by Aniyan et al. [8]. It was higher than findings by Arias-Santiago et al. [7] and others [11,13], yet lower than those by Chalkoo et al. [5].
Deranged HDL (13.2%) was the most common single lipid abnormality, followed by abnormal TC (10.5%), TG (7.9%), deranged VLDL (2.6%). 15.8% of the patients had multiple deranged lipid parameters. This finding differed from that by Aniyan et al. [8] in which deranged VLDL was the most commonly seen, followed by abnormal TG, HDL, and LDL, in that order.
Dyslipidemia was most commonly observed in the 40–49 year-old (31.5%) age group, followed by the 20–29 (21.1%) year-old age group. This was found statistically significant (p = 0.046). Although dyslipidemia is generally more common in the older age group, in this study it was found to be more common in the age group of 40–49 years. This observation agreed with the fact that LP is a disease of the middle-aged population.
Abnormal LDL profile was most common in the 60–79 year-old age group. This finding was statistically significant (p = 0.002). This differed from findings by Aniyan et al. [8] in which the 46–60 year-old age group most commonly had high LDL.
TG abnormality was the highest in the 40–49 year-old age group. This was statistically significant (p = 0.042). Again, this finding differed from that by Aniyan et al. [8] in which the 31–45 year-old age group was most commonly affected.
Although insignificant statistically, the 40–49 year-old age group most commonly had low HDL (p = 0.186). TC abnormality was most common in the 30–59 year-old age group.
There were 37 symptomatic patients, among which HDL was found to be low in 18.4% and abnormal TG, TC, LDL, and VLDL were found in 21.1%, 18.9%, 5.3%, and 5.3% of the patients, respectively. However, none of these findings were statistically significant.
Among the 18.4% of the patients with abnormal HDL, 5.3% had reticular LP, followed by 2.6% each with the plaque, hypertrophic, erosive, follicular, and reactive variants. LDL was found to be high in the reticular and mixed types (2.6% each). VLDL was found to be high in the hypertrophic and reticular types (2.6% each).
Among the 18.9% of the patients with high TC, 10.5%, 5.3%, and 2.6% had the reticular, plaque and erosive types, respectively. 21.1% of the patients had high TG, among whom 7.9% had the reticular variant and 2.6% each had the plaque, hypertrophic, erosive, LPP, and mixed types.
However, the correlation between the clinical types of LP and dyslipidemia was found to be statistically insignificant (Table 6).
CONCLUSION
Dyslipidemia was present in half of the patients with low HDL, being the single most common abnormality. A statistically significant portion of the patients in their 40s had dyslipidemia despite the fact that dyslipidemia is generally more common among the elderly, which may suggest that LP is a disease of the middle age. Statistically significant abnormal TG and LDL were observed in the 40–49 and 60–79 year age groups, respectively. Hence, all patients with LP should be screened for lipid profile abnormalities as a screening procedure for early detection and primary prevention of metabolic syndrome and cardiovascular complications.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Boch K, Langan EA, Kridin K, Zillikens D, Ludwig RJ, Bieber K. Lichen planus. Front Med. 2021;8:737813.
2. Meller S, Gilliet M, Homey B. Chemokines in the pathogenesis of lichenoid tissue reactions. JID. 2009;129:315-9.
3. Toader MP, Taranu T, Constantin MM, Olinici D, Mocanu M, Costan VV, et al. High serum level of interleukin 6 is linked with dyslipidemia in oral lichen planus. Exp Ther Med. 2021;22:1-8.
4. Shenoy C, Shenoy MM, Rao GK. Dyslipidemia in dermatological disorders. North Am J Med Sci. 2015;7:421-8.
5. Chalkoo AH, Peerzada GY, Ahmad MB. Lipid levels in patients with oral lichen planus:A case-control study. IJHSR. 2016;6:180-4.
6. Kar BR, Panda M, Patro N. Metabolic derangements in lichen planus:A case control study. JCDR. 2016;10:WC01-3.
7. Arias-Santiago S, Buendía-Eisman A, Aneiros-Fernández J, Girón-Prieto MS, Gutiérrez-Salmerón MT, Mellado VG, et al. Cardiovascular risk factors in patients with lichen planus. Am J Med. 2011;124:543-8.
8. Aniyan KY, Guledgud MV, Patil K. Alterations of serum lipid profile patterns in oral lichen planus patients:A case–control study. Contemp Clin Dent. 2018;9:S112-21.
9. Manasa D R, Chandru M C, Ravi M Rathod, Vidya Kuntoji. Association of dyslipidemia in lichen planus patients at KIMS, Hubli:A case control study. Med Int J of Biochemistry. 2018;8:1.
10. Kumar SA, Raju PK, Gopal KV, Rao TN. Comorbidities in lichen planus:A case–control study in Indian patients. IDOJ. 2019;10:34-7.
11. López-Jornet P, Camacho-Alonso F, Rodríguez-Martínes MA. Alterations in serum lipid profile patterns in oral lichen planus. Am J Clin Dermatol. 2012;13:399-404.
12. Hashba H, Joy Bifi AT, Sridharan R, Sreenivasan A, Mathew P. Prevalence of metabolic syndrome in patients with lichen planus:A cross-sectional study from a tertiary care center. IDOJ. 2018;9:304-8.
13. Kurian G, Krishnan S, Shakthi P. A prospective case control study on metabolic syndrome in lichen planus in a tertiary care centre. Int J Res Dermatol. 2017;3:427-32.
14. Hammam MA, Bakry OA, Soliman SE, Abd El Hamid EM, Khateeb E. Lipid profile in cases of lichen planus. Menoufia Med J. 2020;33:1050.
15. Khan S, Pirzado MS, Kalhoro HB, Rajper N, Memon SM, Memon FH. Frequency of dyslipidemia in patients with lichen planus:A comparative cross-sectional study. J Liaquat Uni Med Health Sci. 2021;1:1-10.
16. Özkur E, Uğurer E, Altunay İK. Dyslipidemia in lichen planus:A case-control study. Med Bull Sisli Etfal Hosp. 2020;54:62-6.
17. Agarwala MK, Peter D, Thomas N, Paul T, Asha HS, Thomas M, et al. Is lichen planus a marker for dyslipidemia and metabolic syndrome?J Am Acad Dermatol. 2016;74:AB257.
18. Azeez N, Asokan N. Association of lichen planus with dyslipidemia:A comparative, cross-sectional study. Clin Dermatol Rev. 2019;3:68-71.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0009-0008-0791-1081 http://orcid.org/0009-0008-0791-1081 http://orcid.org/0009-0005-9153-0975 http://orcid.org/0009-0005-9153-0975 http://orcid.org/0009-0002-0902- 8029 http://orcid.org/0009-0002-0902- 8029 http://orcid.org/0009-0004-5036-1872 http://orcid.org/0009-0004-5036-1872 http://orcid.org/0009-0007-5309-410X http://orcid.org/0009-0007-5309-410X http://orcid.org/0009-0009-7581-8521 http://orcid.org/0009-0009-7581-8521 |





Comments are closed.