DRESS syndrome and liver involvement: A study of 72 patients
Ghita Sqalli Houssini 1, Zakia Douhi1, Meryem Soughi1, Sara Elloudi1, Hanane Baybay1, Badreddine Moukafih2, Marwa Elbaldi3, Karima Elrhazi3, Fatima Zahra Mernissi1
1, Zakia Douhi1, Meryem Soughi1, Sara Elloudi1, Hanane Baybay1, Badreddine Moukafih2, Marwa Elbaldi3, Karima Elrhazi3, Fatima Zahra Mernissi1
1Department of Dermatology and Venereology, Hassan II University Hospital, Faculty of Medicine and Pharmacy, Sidi Mohammed Ben Abdellah University, Fez, Morocco, 2Central Pharmacy Department, Hassan II university Hospital, Medical Center for Biomedical and Translational Research, Faculty of Medicine and Pharmacy, Sidi Mohammed Ben Abdellah University, Fez, Morocco, 3Laboratory of Epidemiology and Public Health, Faculty of Medicine and Pharmacy, Sidi Mohammed Ben Abdellah University, Fez, Morocco
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: DRESS syndrome (drug reaction with eosinophilia and systemic symptoms syndrome) is a severe drug-induced skin reaction that may be life-threatening, particularly due to its visceral involvement. The liver is the primary organ responsible for the metabolism of most medications. Several reviews and articles have highlighted the liver as the most affected organ in DRESS syndrome, making it intriguing to study this aspect in more detail.
Materials and Methods: This was a retrospective, descriptive, and analytical study conducted at the dermatology department in Fez, Morocco, from 2014 to 2023, including all cases presenting with DRESS syndrome diagnosed based on clinical, biological, histological, and chronological arguments, with a RegiSCAR score classified as probable or definite. Hepatic involvement was assessed based on the classification of drug-induced hepatitis using alkaline phosphatase (ALP) levels, alanine aminotransferase (ALT) levels, and the ALT/ALP ratio (R), thus defining three clinical forms: cytolytic (ALT > 2 or R > 5), cholestatic (ALP > 2 or R < 2), and mixed (2 < R < 5).
Results: 72 patients were included, with 47.2% experiencing hepatic involvement, including 48.5% with cytolytic, 36.4% with cholestatic, and 15% with mixed forms. Among these, 55.9% had associated renal involvement, 29.9% presented with erythroderma, 55.9% with a maculopapular rash, 8.8% with a morbilliform rash, 5.9% with an erythema multiforme-like eruption, and 79.9% had eosinophilia. Allopurinol was the most implicated drug (50%), followed by neuroleptics (17.6%), Salazopyrin (14.7%), and antibiotics (8.8%). The association between Salazopyrin and hepatic involvement was significant (p < 0.05), while the statistical analysis of other parameters did not reveal such an association. Management involved local care, with 50% of the patients placed on corticosteroid therapy. 8.8% of the patients died, while the others showed normalized liver function tests in 74.19% of cases, with the rest lost to follow-up.
Conclusion: Hepatic involvement is common in DRESS syndrome, predominantly manifesting as cytolytic or cholestatic patterns. Maculopapular rash and erythroderma are the most commonly observed cutaneous phenotypes. These patients are more likely to have associated renal involvement and eosinophilia. Allopurinol, neuroleptics, and Salazopyrin are the most frequently implicated drugs.
Key words: DRESS syndrome, Liver, Hepatic, Allopurinol, Drug
INTRODUCTION
DRESS syndrome is a severe drug reaction defined by a clinical and biological presentation that includes high fever, facial edema, skin rash, polyadenopathy, mononucleosis-like syndrome, eosinophilia, and visceral involvement [1]. Its pathophysiology has become clearer with the identification of viral reactivations, including human herpesvirus 6 (HHV-6), human herpesvirus 7 (HHV-7), cytomegalovirus (CMV), and Epstein–Barr virus (EBV) [2]. Its severity is associated with systemic manifestations that may progress to multi-organ failure, jeopardizing the prognosis [3]. Hepatic involvement is well-documented in DRESS syndrome, ranging from a simple transient abnormality in liver function tests to severe hepatic failure, known as drug-induced liver injury (DLI), an idiosyncratic drug-related liver injury [4,5]. Drug-induced hepatotoxicity remains challenging to ascertain due to the absence of diagnostic criteria and specific biomarkers. However, in cases of suspected drug involvement and the absence of viral or autoimmune causes, the likelihood of drug-induced hepatotoxicity remains highly probable [5].
MATERIALS AND METHODS
Study Design
We conducted a retrospective, descriptive, and analytical study within the dermatology department of Hassan II University Hospital in Fes, Morocco, over a period of nine years. We included all patients hospitalized for DRESS syndrome. The diagnosis was based on clinical criteria (skin rash), biological criteria (eosinophilia, lymphopenia, or leukocytosis, alteration of renal or hepatic function), histological criteria through a skin biopsy, chronological criteria (time between drug intake and symptomatology), with reporting to our institution’s pharmacovigilance center. The RegiSCAR score was calculated for all our patients and categorized as probable or definite. Other cases of severe drug reactions were excluded.
Hepatic involvement was either incidentally discovered based on biochemical abnormalities or during clinical signs such as jaundice or asthenia. Causality assessment relied primarily on chronological and clinical criteria, eliminating other potential causes and demonstrating the suspected drug’s role [6]. The classification of the type of hepatitis was defined based on alkaline phosphatase (ALP), alanine aminotransferase (ALT) levels, as well as the ALT(N)/ALP(N) ratio; N = upper limit: cytolytic injury (ALT > 2N or ratio > 5), cholestatic injury (ALP > 2N or ratio < 2), and mixed injury (2 < ratio < 5) [6].
We studied the incidence of hepatic involvement and the principal clinical, biological, and evolutionary characteristics in patients with this dysfunction: type of skin rash, eosinophilia, renal failure, implicated drugs, and vital prognosis.
Analytical Study
Our data was analyzed with SPSS software, version 26, and descriptive results were reported as valid percentages (%). The chi-squared test and Fisher’s test were employed to explore significant correlations between hepatic involvement and the various parameters studied. The result was considered significant if the p value was less than 0.05. The p value could not be calculated in certain cases and we designated these results as (-).
RESULTS
Epidemiological Data
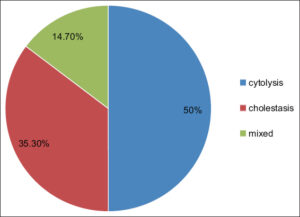
We collected data from 72 patients diagnosed with DRESS syndrome. The average age was 56 years, and the sex ratio (M/F) was 0.6. Hepatic involvement was observed in 34 patients (47.2%), primarily detected through laboratory tests. Additionally, 3 patients (4%) presented with cutaneous-mucosal jaundice associated with asthenia. Drug-induced hepatitis manifested as cytolytic in 17 cases (50%), cholestatic in 12 (35.3%), and mixed in 5 (14.7%) (Fig. 1). Patients with hepatic involvement had an average age of 53 years, with a female predominance of 61.8%, and a history of diabetes (17.6%), renal insufficiency (2.9%), and cardiovascular abnormalities (41.2%). No significant differences were found in age, sex, or medical history between patients with and without hepatic involvement.
Clinical and Systemic Manifestations and Drugs Involved
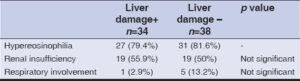
Among patients with hepatic involvement, 19 (55.9%) had a maculopapular rash (Fig. 2a), 10 (29.4%) had erythroderma (Fig. 2b), 3 (8.8%) had morbilliform rash, and 2 (5.9%) had polymorphic erythema described as a diffuse rash associated with target lesions and/or pseudo-target lesions (Fig. 2c). Notably, 19 patients (55.9%) had mucosal involvement (Fig. 2d). There was no significant association between the type of skin eruption and hepatic involvement. Clinical characteristics of patients with hepatic impairment are summarized in Table 1.
 |
Figure 2: Clinical photos of different skin patterns: a) maculopapular rash, b) erythroderma, c) erythema multiforme-like, d) mucosal involvement. |
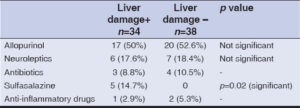
Regarding systemic manifestations in patients with hepatic involvement, 27 cases (79.4%) had eosinophilia, 19 (55.9%) had renal insufficiency, and 1 (2.9%) had respiratory involvement. No significant association was found between systemic involvement and hepatic impairment. Systemic manifestations are summarized in Table 2. The most implicated drugs, in order of frequency, were allopurinol (50%), followed by neuroleptics (17.6%), sulfasalazine (14.7%), and antibiotics (8.8%). The association between sulfasalazine and hepatic involvement was significant (p < 0.05), while the association between other therapeutic classes and hepatic impairment did not show a significant correlation. Incriminated drugs in patients with and without hepatic impairment are summarized in Table 3.
Treatment and Prognosis
Therapeutic management locally was consistent regardless of hepatic abnormality, primarily involving local care, initiation of topical corticosteroids compounded or in a 30 g protocol. Among all patients with DRESS syndrome, 22 cases (30.6%) were treated with injectable corticosteroids in mini-boluses of 0.5 mg/kg of methylprednisolone, followed by oral administration. Notably, 17 patients (77.2%) with corticosteroid therapy experienced hepatic involvement. Concerning prognosis, 3 patients (8.8%) with hepatic abnormalities died. Among these three deceased patients, two had cytolytic patterns, and one exhibited cholestasis. The causes of death were sepsis, end-stage renal failure, and multiorgan failure in a patient with a history of severe cardiovascular disease. The remaining patients showed normalization of hepatic parameters in 74.19%, while the rest were lost to follow-up.
DISCUSSION
DRESS syndrome is a delayed drug hypersensitivity reaction occurring 2 to 6 weeks after taking the medication. It is a severe drug eruption that may have life-threatening consequences. Its clinical and biological characteristics are now well-known, enabling its identification. The diagnosis relies on a triad, including a skin rash, hematological abnormalities such as eosinophilia or atypical lymphocytosis, and visceral involvement, notably affecting the liver and kidneys. The liver is the most commonly affected organ in DRESS syndrome [4,7–9]. This involvement, or what we may call drug-induced liver injury (DLI), may range from a simple biological disturbance to hepatic failure [7]. Indeed, the liver is the organ where the metabolism of several drugs takes place. The diagnosis of drug-induced hepatitis remains a challenge for hepatologists due to the lack of standardized diagnostic criteria and reliable biological markers [5]. Various definitions and upper limits for AST, ALT, alkaline phosphatase, or bilirubin levels exist. Considering hepatic tolerance that may occur, some authors suggest diagnosing drug-induced hepatitis if transaminase levels exceed five times the normal without clinical signs, or if alkaline phosphatase levels are more than two times the normal, or if bilirubin is more than two times the normal [10]. We based our definition on the initial one, which was also adopted by I-Chun Lin et al. in their retrospective study involving 72 patients [8]. This definition, chosen by hepatologists at our university hospital, guided our approach [6].
Moreover, our results showed that the liver is frequently affected (47.2%), which was in line with the findings by Lee et al. (45%) [9]. It is noteworthy that a literature review on liver involvement in DRESS syndrome conducted by Sylvia A Martinez-Cabriale et al. asserts that the liver is the most affected organ in DRESS syndrome, with frequencies varying among authors from 51% to 87% [7]. The pathophysiology seems to be explained by the viral reactivation of HHV6 that occurs during DRESS syndrome and may lead to hepatitis. Another hypothesis is related to the infiltration of eosinophils secondary to excessive inflammatory reaction and the influx of IL-5 during DRESS syndrome [7].
The most noted form of drug-induced liver injury (DLI) in our series was the cytolytic form (50%), followed by the cholestatic form (35.3%) and the mixed form (14.7%). Indeed, according to Sylvia A Martinez-Cabriales et al., acute hepatitis is often either cytolytic or cholestatic depending on the age of the patient and the implicated drugs [7,8]. It seems that cytolytic involvement is more common in younger subjects under antibiotics and carbamazepine, whereas older subjects under allopurinol or phenytoin tend to present the cholestatic form [8]. This aligns with our results, as our patients with liver involvement had an average age of 53 years, relatively young, which explains the predominant cytolytic involvement in our series. On the other hand, allopurinol was the most incriminated drug in our study, which may be explained by the fact that the majority of our patients had comorbidities such as cardiovascular history, diabetes, or renal insufficiency, making allopurinol a commonly prescribed medication by general practitioners, cardiologists, or nephrologists. Furthermore, our results showed a significant association between the use of sulfasalazine and liver involvement. The involvement of sulfasalazine in DRESS syndrome has been reported in the literature in several case reports [11–13]. Its association with drug-induced hepatic injury has also been documented [14], both in DRESS syndrome and in acute generalized exanthematous pustulosis [14,15]. A study reported that beta-lactam antibiotics, allopurinol, non-steroidal anti-inflammatory drugs, and sulfamides were the major contributors to DRESS syndrome with liver involvement [9]. Another study reported that sulfamides (92.9%), followed by antiepileptics (86.3%) and allopurinol (78%), presented the highest risk of inducing liver damage in DRESS syndrome [8].
Regarding associated clinical features, our series showed that the maculopapular rash was the most observed (55.9%) in patients with liver involvement, followed by erythroderma (29.4%), morbilliform exanthem (8.8%), and erythema polymorphe-like (5.9%), defined by the presence of a rash and lesions in a target or pseudo-target pattern. Walash et al., in their series of 27 patients with DRESS syndrome, demonstrated that erythema polymorphe-like and the presence of purpura were associated with more severe liver involvement when compared to other types of eruptions [16]. However, defining a cutaneous phenotype as a prognostic marker for visceral involvement remains a subject of controversy, and a study by Kettani et al. did not show a significant association in this regard [17].
Regarding systemic manifestations, Lee et al. reported that renal dysfunction was more frequent in patients with hepatic dysfunction (39% vs. 1%, p = 0.001), and patients with hepatic dysfunction were more likely to have renal dysfunction (96% vs. 34%, p = 0.001) [18]. This aligned with our results, in which renal insufficiency was more common in patients with liver involvement (55.9%) when compared to those without involvement (50%).
Therapeutic management is not well standardized, and the utility of systemic corticosteroid treatment is a subject of debate. A study by Lee et al. demonstrated that, in patients with DRESS syndrome and hepatic involvement, the use of systemic corticosteroids did not provide additional benefits in terms of disease duration and improvement in liver function [9]. Furthermore, a favorable outcome was reported in a study by Decloux et al., in which fulminant hepatitis was treated with methylprednisolone 1 g/day for three days, followed by a prolonged course of prednisone (3750 mg over thirty days) [19]. It is interesting to note that some authors observed relapses after tapering corticosteroids and had to resume oral corticotherapy at higher doses, highlighting once again that DRESS syndrome is a chronic inflammatory syndrome with an unpredictable long-term course [4].
The mortality rate in DRESS syndrome ranges from 5% to 10%, which is primarily attributed to severe involvement of the internal organs such as the liver, heart, lungs, and kidneys. Severe forms, such as hepatic encephalopathy or fulminant hepatitis requiring urgent liver transplantation, have a poor prognosis and significant morbidity and mortality [20]. In our series, the vast majority of the patients presented with an asymptomatic form of drug-induced liver injury (DLI), explaining the reduced mortality and subsequent normalization of their biological parameters. In their case series, Ichai et al. found that 43% (7/16) of patients with hepatic involvement during DRESS syndrome either underwent transplantation (n = 5) or died (n = 2) [20]. DILI stands as a crucial contributor to acute liver failure, carrying substantial morbidity and mortality implications. Each medication exhibits a distinctive pattern of liver injury, and the prognosis varies accordingly. In addition to considering the specific drug type, factors such as age, bilirubin levels, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and prothrombin time (PT) are assessed as indicators of mortality. Despite this, the precise thresholds for bilirubin, PT, or other factors that reliably predict the severity or mortality risk in patients with drug-induced liver injury (DILI) remain undefined. In their study, Sunil Kumar et al. found that patients with DRESS syndrome exhibited less severe liver impairment when compared to those without DRESS syndrome [21]. However, prompt management involving the immediate cessation of the causative drug and the identification of systemic involvement, especially in the liver, could lead to a better prognosis for the patient. The benefit of high-dose systemic corticosteroid treatment remains a subject of controversy.
Study Limitations
Our study had several limitations. Despite relying on the meticulous registry of the adverse drug reactions database from a single medical center, our university hospital, in close collaboration with the pharmacovigilance center, the number of cases was limited. This limitation hindered our ability to draw didactic comparisons and obtain statistically significant results. All our findings demonstrated a non-significant association, except for the association between sulfasalazine and hepatic involvement. Finally, a comprehensive interpretation of our results would require a better understanding of the underlying pathological mechanisms of liver lesions. A large prospective study would be essential to address these questions, particularly regarding the various clinical and biological characteristics and the management of patients with hepatic involvement in DRESS syndrome.
CONCLUSION
The liver is the most affected organ in DRESS syndrome, often presenting as asymptomatic drug-induced liver injury (DLI) and rarely as severe fulminant hepatitis. The clinical form of hepatitis varies with age and the implicated drug, and the associated clinical and biological characteristics remain a subject of ongoing research. Our series demonstrated a predominance of cytolytic hepatitis in a relatively young population, primarily associated with allopurinol. We found a significant association between Salazopyrin and hepatic dysfunction. The correlation between cutaneous phenotype and hepatic involvement is variable, with patients having hepatic dysfunction being more prone to developing renal insufficiency. High-dose corticosteroids appear to be beneficial for some, yet for others, there is no observed benefit in terms of the severity of hepatic involvement.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Descamps V, Mardivirin L, Janela B, Musette P, Ranger-Rogez S. Le syndrome d’hypersensibilité(DRESS) n’est qu’une maladie virale. Rev Fr Allergol. 2010;50:171-3.
2. Descamps V, Ranger-Rogez S, Musette P, Barbaud A. Le DRESS (drug reaction with eosinophilia and systemic symptoms):une synergie médicaments-virus qui peut conduire en réanimation. Réanimation. 2011;20:223-7.
3. Chebbi W, Souissi J, Chelli J, Larbi F, Zantour B, Habib Sfar M. DRESS syndrome:Report of three cases. Pan Afr Med J. 2014;19:166.
4. Lens S, Crespo G, Carrión JA, Miquel R, Navasa M. Severe acute hepatitis in the DRESS syndrome:Report of two cases. Ann Hepatol. 2010;9:198-201.
5. Devarbhavi H. An Update on Drug-induced liver injury. J Clin Exp Hepatol. 2012;2:247-59.
6. Abid H, Lahmidani N, El yousfi M, Benajah D, El abkari M, Aqodad N. Le point sur l’hépatite médicamenteuse. RMG. 2019;10:100-6.
7. Martinez-Cabriales SA, Shear NH, Gonzalez-Moreno EI. Liver involvement in the drug reaction, eosinophilia, and systemic symptoms syndrome. World J Clin Cases. 2019;7:705-16.
8. Lin IC, Yang HC, Strong C, Yang CW, Cho YT, Chen KL. Liver injury in patients with DRESS:A clinical study of 72 cases. J Am Acad Dermatol. 2015;72:984-91.
9. Lee T, Lee YS, Yoon SY, Kim S, Bae YJ, Kwon HS. Characteristics of liver injury in drug-induced systemic hypersensitivity reactions. J Am Acad Dermatol. 2013;69:407-15.
10. Fontana RJ, Seeff LB, Andrade RJ, Björnsson E, Day CP, Serrano J, et al. Standardization of nomenclature and causality assessment in drug-induced liver injury:Summary of a clinical research workshop. Hepatology. 2010;52:730-42.
11. Kanabaj K, Jenerowicz D, Jankowska L, Żaba Z. DRESS syndrome:A dermatological emergency:Sulfasalazine-related acute drug reaction case report. Heliyon. 2023;9:e20021.
12. Michel F, Navellou JC, Ferraud D, Toussirot E, Wendling D. DRESS syndrome in a patient on sulfasalazine for rheumatoid arthritis. Joint Bone Spine. 2005;72:82-5.
13. Girelli F, Bernardi S, Gardelli L, Bassi B, Parente G, Dubini A et al. A new case of DRESS syndrome induced by sulfasalazine and triggered by amoxicillin. Case Rep Rheumatol. 2013;2013:409152.
14. Jennings PE, Blandford RL, Rosenthal FD. Acute sulphasalazine hepatotoxicity. Postgrad Med J. 1986;62:305-6.
15. Białynicki-Birula R, CisońW, Krefft-Trzciniecka H, CisońH. Acute generalized exanthematous pustulosis caused by sulfasalazine. Our Dermatol Online. 2024;15:71-74.
16. Walsh S, Diaz-Cano S, Higgins E, Morris-Jones R, Bashir S, Bernal W. Drug reaction with eosinophilia and systemic symptoms:Is cutaneous phenotype a prognostic marker for outcome?A review of clinicopathological features of 27 cases. Br J Dermatol. 2013;168:391-401.
17. Kettani F, Hali F, Baline K, Chiheb F. Dress syndrome :phénotype cutanéet atteinte viscérale (étude rétrospective de 62 cas). Rev Fr Allergol. 2020;60:69-74.
18. Lee WM, Squires RH Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure:Summary of a workshop. Hepatology. 2008;47:1401-15.
19. Descloux E, Argaud L, Dumortier J, Scoazec JY, Boillot O, Robert D. Favourable issue of a fulminant hepatitis associated with sulfasalazine DRESS syndrome without liver transplantation. Intensive Care Med. 2005;31:1727-8.
20. Ichai P, Laurent-Bellue A, Saliba F, Moreau D, Besch C, Francoz C. Acute liver failure/injury related to drug reaction with eosinophilia and systemic symptoms:Outcomes and prognostic factors. Transplantation. 2017;101:1830-7.
21. Sunil Kumar N, Remalayam B, Thomas V, Ramachandran TM, Sunil Kumar K. Outcomes and predictors of mortality in patients with drug-induced liver injury at a tertiary hospital in south India:A Single-Centre Experience. J Clin Exp Hepatol. 2021;11:163-70.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0009-0008-4480-2968 http://orcid.org/0009-0008-4480-2968 http://orcid.org/0000-0002-5942-441X http://orcid.org/0000-0002-5942-441X http://orcid.org/0000-0003-3455-3810 http://orcid.org/0000-0003-3455-3810 |





Comments are closed.