Cutaneous rash in patients receiving imatinib: Lichenoid drug eruption or induced lichen?
Soukaina Karimi , Maryem Aboudourib, Said Amal, Ouafa Hocar
, Maryem Aboudourib, Said Amal, Ouafa Hocar
Dermatology and Venerology Department, Arrazi Hospital, The Mohammed VI universal Hospital, Marrakesh, Morocco
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
Sir,
Imatinib is a tyrosine kinase receptor inhibitor used in the treatment of chronic myeloid leukemia, gastrointestinal stromal tumors, and Darier–Ferrand dermatofibrosarcoma.
Several cutaneous side effects were described with this molecule, including lichenoid eruptions, which remain rare. Herein, we report a new observation.
A 61-year-old female patient had a history of chronic myeloid leukemia and was put on imatinib by her hematologist. She received 400 mg per day for six months with good tolerance so far. The patient presented with a generalized itchy rash persistent for a month.
A dermatological examination revealed general xerosis and several erythematous and squamous plaques that were well-limited, shiny, and lichenified in some areas. Cutaneous lesions were of different sizes, sometimes confluent and symmetrically distributed on the trunk, arms, and thighs sparing the face (Figs. 1a and 1b).
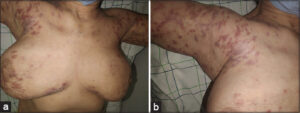 |
Figure 1: (a) Multiple, symmetrical, erythematous and squamous plaques on the trunk and upper arm. (b) Erythematous and squamous plaques on the shoulder (note the shiny and purplish aspect). |
An examination of the oral mucosa showed enanthema with a fine reticulated network of the inner surface of the right cheek.
A skin biopsy revealed orthokeratotic hyperkeratosis, necrotic keratinocyte cells (Civatte bodies), pigmentary incontinence, and a dense lichenoid lymphoid infiltrate consisting of lymphocytes and eosinophils. In addition, blood work found no abnormalities and hepatitis B and C serologies were negative.
Therapeutic management consisted of an emollient, antihistamines, and a local corticosteroid for the skin and oral lesions.
A follow-up after eight weeks of treatment noted the disappearance of pruritus, the absence of new lesions, post-inflammatory hyperpigmentation (Fig. 2). Imatinib was continued at the same dose of 400 mg per day. No recurrence was observed for two years (Fig. 3).
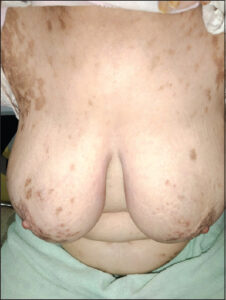 |
Figure 2: Follow-up after eight weeks of local treatment (note post-inflammatory pigmentation). |
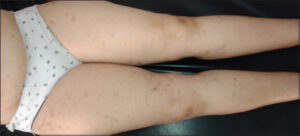 |
Figure 3: Follow-up at six months noted no new lesions and clarification of the hyperpigmentation. |
The cutaneous side effects of imatinib are frequent and occur between 9% and 69% [1]. They include xerosis, alopecia, facial edema, and photosensitivity [2]. Lichenoid drug eruption under imatinib is rare, with around thirty published cases [3]. The period between imatinib initiation and the rash onset typically ranges from one to twelve months [2].
The clinical presentation of this drug eruption is polymorphic, associating psoriasiform, lichen-like, and sometimes even eczema-like lesions. Oral lesions manifest with erythema or ulcerated whitish plaques that may have a reticulated form and linear streaks [4].
A distinction between a lichenoid drug eruption and induced lichen planus may be challenging. Clinically, lichenoid drug eruption is associated with a more symmetrical arrangement of lesions on the trunk and extremities, located on photo-exposed areas, without Wickham’s streaks and evolving toward post-inflammatory hyperpigmentation. Meanwhile, histology rather shows focal parakeratosis and eosinophilic infiltrate with a perivascular arrangement [5].
In our case, there was an overlap between the clinical and histological appearance of these two entities. Similar observations have been reported in the literature.
Imatinib withdrawal is not mandatory, and dose reduction with a local symptomatic treatment based on topical corticosteroids usually allows improvement. In more resistant cases, other treatments are suggested. Dalmau et al. recommended acitretin [4].
Imatinib-induced lichenoid drug eruption is generally benign. However, severe or treatment-resistant forms require dose reduction or even treatment discontinuation, which risks aggravating the underlying pathology. The definitive discontinuation of imatinib should, therefore, be discussed based on the benefit/risk ratio of each patient.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Bouabdella S, Sof K, Khouna A, Zizi N, Dikhaye S. Éruptions lichénoides induites par l’Imatinib:A propos de deux cas. Presse Médicale Form. 2021;2.
2. Penn EH, Chung HJ, Keller M. Imatinib mesylate-induced lichenoid drug eruption. Cutis. 2017;99:189-92.
3. Frioui R, Faten R, Asma B, Jaber K, Dhaoui MR. Toxidermies lichénïdes sous inhibiteurs des tyrosines kinases:un effet indésirable àne pas méconnaître. Rev Méd Interne. 2021;42:A412.
4. Meddeb Z, Souissi A, Derbel F, Bellakhal S, Jellouli A, Mestiri A, et al. La toxidermie lichénoïde àl’imatinib:un effet indésirable àne pas méconnaître. Rev Méd Interne. 2015;36:A867.
5. Pascual JC, Matarredona J, Miralles J, Conesa V, Borras-Blasco J. Oral and cutaneous lichenoid reaction secondary to imatinib:Report of two cases. Int J Dermatol. 2006;45:14713.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (contact@odermatol.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|




Comments are closed.