Rifampicin-induced Sweet’s syndrome following erythema induratum of Bazin
Chaymae Jroundi , Hanane Baybay, Jihad Kassel, Zakia Douhi, Sara Elloudi, Fatima Zahra Mernissi
, Hanane Baybay, Jihad Kassel, Zakia Douhi, Sara Elloudi, Fatima Zahra Mernissi
Department of Dermatology, University Hospital Hassan II, Fes, Morocco
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
Sir,
Sweet’s syndrome is a neutrophilic dermatosis characterized by abrupt infiltration of the superficial dermis by neutrophils in the absence of local infection. It is classified as classic, malignancy-induced, or drug-induced [1,2]. Medications are responsible for approx. 12% of Sweet’s syndrome presentations with granulocyte colony-stimulating factor, all-trans retinoic acid, and vaccines cited as the most common causes [3]. Unlike the rest of antituberculous drugs, rifampicin is usually well tolerated by most patients, although severe digestive intolerance sometimes requires the discontinuation of the treatment. Cutaneous adverse effects have been described, including occasional flushing and maculopapular exanthema [4]. Herein, we report a case of rifampicin-induced Sweet’s syndrome in a patient with erythema induratum of Bazin (EIB).
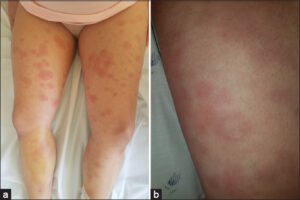
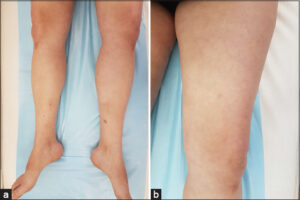
A 45-year-old female patient, with a history of chronic, recurrent, nodular lesions on the lower limbs treated as erythema nodosum, was treated with non-steroidal, anti-inflammatory drugs, then corticosteroids, without improvement. She had no personal or family history of active tuberculosis. The diagnosis of EIB was, then, reached based on the clinical morphology and evolution, histopathology, a tuberculin test, and QuantiFERON-B Gold Plus were also all positive. Antituberculous (ATB) treatment was then initiated. The patient reported a cutaneous rash ten days after the initiation of the treatment. All ATB drugs were stopped and reintroduced progressively with a two-week interval, starting with isoniazid (H), ethambutol (E), pyrazinamide (Z), and lastly rifampin (R) at a dose of 150 mg. After the reintroduction of rifampicin, the patient developed malaise, fever, chills, and arthralgia. Two days later, a tender non-pruritic rash characterized by painful, swollen, erythematous plaques and subcutaneous nodules symmetrically distributed on the thighs, legs, and upper arms was observed (Figs. 1a and 1b), leading to the discontinuation of the treatment by the patient. Biological assessment revealed hyperleukocytosis (14.2 × 103/mm3) with 80% of neutrophils, an inflammatory syndrome with a high level of C-reactive protein (at 228), and the erythrocyte sedimentation rate at 42. A diagnosis of Sweet’s syndrome was confirmed by the histological findings of a dense dermal neutrophilic infiltrate extending deeper to the hypodermis in a lobular distribution, focal leukocytoclasia, and papillary dermal edema. After a consultation with pneumoallergists, the patient was put back on ATB with progressive administration. Along with close monitoring, oral corticosteroids were initiated at a dose of 1 mg/kg/day with tapering over six months. A marked improvement occurred over the next month with the regression of EIB lesions. A minimal flare-up following the reintroduction of rifampicin was noted, characterized by painful, swollen plaques in the legs, rapidly resolving after three days, and a complete regression of all lesions occurred three months later (Figs. 2a and 2b).
Along with the clinical and histopathological description, a temporal relationship between drug ingestion and the onset of symptoms as well as the resolution of symptoms after drug withdrawal and treatment with systemic steroids confirmed the diagnosis of rifampicin-induced Sweet’s syndrome in our patient.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Shaikh MB, Krunic AL. Drug-induced Sweet syndrome. J Allergy Clin Immunol Pract. 2019;7:2400-1.
2. Belmourida S, Khallayoune M, Palamino H, Meziane M, Ismaili N, Benzekri L, Hassam B, Senouci K. Sweet syndrome revealing a leukemia. Our Dermatol Online. 2020;11:e139.1-2.
3. McNally A, Ibbetson J. Azathioprine-induced Sweet’s syndrome:A case series and review of the literature. Australas J Dermatol. 2017;58:53-7.
4. Campbell JR, Trajman A. Adverse events in adults with latent tuberculosis infection receiving daily rifampicin or isoniazid:Post-hoc safety analysis of two randomised controlled trials. Lancet Infect Dis. 2020;20:318-29.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (contact@odermatol.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-3455-3810 http://orcid.org/0000-0003-3455-3810 http://orcid.org/0000-0002-5942-441X http://orcid.org/0000-0002-5942-441X |




Comments are closed.