Ibrutinib-induced pyoderma gangrenosum in a patient with chronic lymphocytic leukemia
Insaf Moubine 1, Fouzia Hali1, Fatima Zahra El Fatoiki1, Meryem Azim2, Farida Marnissi2, Asmae Meftah3, Houda Filali3, Mouna Lamchahab4, Soumiya Chiheb1
1, Fouzia Hali1, Fatima Zahra El Fatoiki1, Meryem Azim2, Farida Marnissi2, Asmae Meftah3, Houda Filali3, Mouna Lamchahab4, Soumiya Chiheb1
1Department of Dermatology and Venereology, Ibn Rochd University Hospital, Casablanca, Morocco, 2Department of Anatomy Pathology, Ibn Rochd University Hospital, Casablanca, Morocco, 3Department of Pharmacology, Faculty of medicine and pharmacy, Casablanca, Morocco, 4Department of Clinical Hematology, 20 Aout University Hospital, Casablanca, Morocco
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
Sir,
Pyoderma gangrenosum (PG) is a rare neutrophilic dermatosis of undetermined etiology. The pathophysiological mechanism is not fully understood. It is most often associated with systemic diseases: inflammatory bowel diseases, hematologic disorders, and solid tumors [1]. Drug-induced PG is an uncommon cutaneous reaction with no specific clinical or histopathologic features [2]. Herein, we describe a new case of ibrutinib-induced PG in a patient with B-cell chronic lymphocytic leukemia.
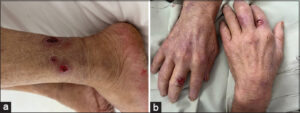
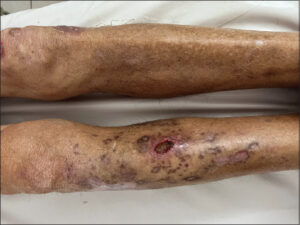
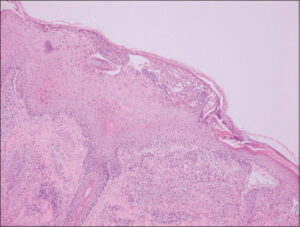
A 64-year-old male with a history of B-cell chronic lymphocytic leukemia was diagnosed three years earlier, treated with chemotherapy for two years, and achieved an incomplete remission. Thus, he was started on the Bruton tyrosine kinase inhibitor ibrutinib at the recommended dose of 420 mg/day with significant improvement. After eight months, the patient consulted for several painful, palpable, red-to-violaceous skin lesions in the lower limbs and feet. The lesions began one month after ibrutinib initiation with a vesicular appearance and evolved into nodules and painful ulcers with violaceous borders, sometimes associated with pustules (Figs. 1a and 1b). He also presented dyschromic and cribriform scars on his right leg (Fig. 2). A biopsy of the ulcer margin and pustules revealed massive lymphocyte and neutrophil infiltration with few apoptotic bodies. No histopathologic signs of vasculitis were observed (Fig. 3). The complete blood count did not reveal any leukocytosis, and the bacterial and fungal cultures were negative. After consultation with hematologists and pharmacologists, PG was diagnosed, and ibrutinib was suspected as the causative agent. In addition to ibrutinib discontinuation, the patient received topical corticosteroids, and an improvement in the skin lesions was observed.
PG is a neutrophilic dermatosis clinically defined by painful ulcers with a violaceous border, usually located in the lower limbs. It may also appear as aseptic pustules or, less frequently, nodules [1]. Histopathology is not specific; its main purpose is to exclude other potential diagnoses. PG typically imitates an abscess or cellulitis by displaying considerable neutrophilic infiltration, bleeding, and epidermal necrosis. Sometimes, vessel wall infiltration may be noticed [3]. Innovative therapies that focus on B-cell receptors and their signaling pathways are quickly improving the treatment options for B-cell malignancies. Ibrutinib is an example of a drug that has been approved for the treatment of mantle cell lymphoma, chronic lymphocytic leukemia, and other malignant hematologic conditions. It acts by inhibiting Bruton tyrosine kinase (BTK), which is necessary for B cells to survive and reproduce [4]. The most commonly reported side effects are cytopenia, diarrhea, fatigue, bruising, and upper respiratory tract infections. Only several cases of ibrutinib-induced neutrophilic dermatosis, including two cases of PG, have been reported in the literature [5,6]. Ibrutinib has a good safety profile and few adverse effects because of its BTK receptor specificity. The epidermal growth factor receptor, which is expressed in the basal layer of the epidermis, is another target of its function. Inhibition of this receptor has a negative effect on tissue regeneration and is responsible for a pro-inflammatory reaction that may explain the occurrence of PG, which is more common in areas susceptible to repeated trauma [3]. Another theory contends that host immune cells are exposed to peptides conjugated to ibrutinib through the major histocompatibility complex, which triggers a T cell-directed immune response leading to tissue damage and destruction [2].
Ibrutinib, a BTK inhibitor, could lead to neutrophilic dermatosis, which is explained by the drug-induced immune process. Ibrutinib-dose tapering, low-dose corticosteroids, and dapsone remain the most effective options to treat this condition and prevent recurrence. Switching from ibrutinib to another medication could be suggested in the case of resistance.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Kridin K, Cohen AD, Amber KT. Underlying systemic diseases in pyoderma gangrenosum:A systematic review and meta-analysis. Am J Clin Dermatol. 2018;19:479-47.
2. Khoshnam-Rad N, Gheymati A, Jahangard-Rafsanjani Z. Tyrosine kinase inhibitors-associated pyoderma gangrenosum:A systematic review of published case reports. Anti-Cancer Drugs. 2022; 33:e1-e8.
3. Marzano AV, Ishak RS, Saibeni S, Crosti C, Meroni PL, Cugno M. Autoinflammatory skin disorders in inflammatory bowel diseases, pyoderma gangrenosum and Sweet’s syndrome:A comprehensive review and disease classification criteria. Clin Rev Allergy Immunol. 2013;45:202-10.
4. Shaikh H, Khattab A, Faisal MS, Chilkulwar A, Albrethsen M Sadashiv S, et al. Case series of unique adverse events related to the use of ibrutinib in patients with B-cell malignancies:A single institution experience and a review of literature. J Oncol Pharm Practice. 2018;0:1-6.
5. Giovanni B, Ibatici A, Sola S, Maria A, Brunasso G, Massone C. Ibrutinib and pyoderma gangrenosum in a patient with b-cell chronic lymphocytic leukemia. Am J Dermatopathol. 2020;42:148-50.
6. Sławińska M, Barańska-Rybak W, Sobjanek M. Ibrutinib-induced pyoderma gangrenosum. Pol Arch Med Wewn. 2016;126:710-11.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (contact@odermatol.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0001-5407-0184 http://orcid.org/0000-0001-5407-0184 http://orcid.org/0000-0001-6868-892X http://orcid.org/0000-0001-6868-892X http://orcid.org/0000-0002-9475-2785 http://orcid.org/0000-0002-9475-2785 http://orcid.org/0000-0003-1011-3271 http://orcid.org/0000-0003-1011-3271 http://orcid.org/0000-0001-9120-6845 http://orcid.org/0000-0001-9120-6845 http://orcid.org/0000-0002-6206-9981 http://orcid.org/0000-0002-6206-9981 |




Comments are closed.