Favorable evolution of acute erythema nodosum leprosum under prednisolone and clofazimine at Dosso Regional Hospital in Niger
Moussa Harouna 1, Kadidia Issa Abdou2, Abdoul Kadir Ibrahim Mamadou3, Saraye Ousmane4, Mazou Hamadou5, Idrissa Boubacar6, Oumalkhair Sidi Zakari1
1, Kadidia Issa Abdou2, Abdoul Kadir Ibrahim Mamadou3, Saraye Ousmane4, Mazou Hamadou5, Idrissa Boubacar6, Oumalkhair Sidi Zakari1
1Dermatology and Venereology Department of Dosso Regional Hospital, Niger, 2Department of Dermatology and Venereology of Zinder National Hospital, Niger, 3Internal Medicine Department of Dosso Regional Hospital, Niger, 4Department of Dermatology and Venereology of Niamey National Hospital, Niger, 5Pediatrics Department of Maradi Regional Hospital, Niger, 6Anatomy Pathology and Cytology Department of Niamey National Hospital, Niger
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
During the course of leprosy, some patients will develop a reaction. Acute inflammatory events occur in approx. 25% of patients, usually during and sometimes at the end of treatment. There are two types of a leprosy reaction: type I or reverse reaction, and type II or erythema nodosum leprosum (ENL). It is a therapeutic emergency, and recurrence is frequent. ENL is treated with corticosteroids and other immunosuppressants such as thalidomide, azathioprine, and cyclosporine. Some patients respond favorably to treatment, while others become refractory. Herein, we report the case of a 49-year-old female with acute ENL treated with prednisolone and clofazimine for twelve months with a favorable evolution.
Key words: Leprosy, Leprous reaction, Corticosteroids, Clofazimine, Dosso
INTRODUCTION
Leprosy is a chronic, debilitating infection, known since ancient times, caused by Mycobacterium leprae. It is a slow-multiplying, in vitro non-cultivable germ with cutaneous and peripheral nerve tropism. Acute inflammatory manifestations occur in around 25% of patients, generally during and sometimes after treatment. A distinction is made between type 1, or reverse reaction, and type 2, or erythema nodosum leprosum (ENL), whose manifestations are varied, combining fever, painful acute neuritis with paralysis, edema, and redness of pre-existing skin lesions. It is a disseminated nodular erythema nodosum and is inflammatory, hence sensitive, and associated with uveitis, polyarthritis, adenitis, and orchiepididymitis. It constitutes a therapeutic emergency, and recurrence is frequent [1]. ENL affects around 50% of people with lepromatous leprosy and 5–10% of patients with lepromatous leprosy [2]. In the majority of patients, ENL is a chronic disease requiring prolonged immunosuppression. Thalidomide is effective in controlling ENL and is recommended by the WHO under strict medical supervision due to its serious teratogenic effects. However, it is not available in numerous countries where leprosy is endemic. Patients must, therefore, take high doses of oral corticosteroids, often for many years. The WHO recommends a high dose of clofazimine in combination with prednisolone for the management of severe ENL [3].
Our work was to report a case of acute ENL in a 49-year-old patient followed at our department with a favorable evolution.
CASE REPORT
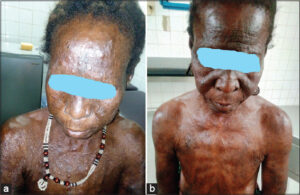
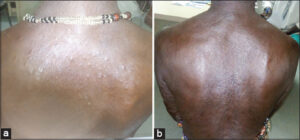
A 49-year-old housewife with a history of multibacillary leprosy with grade II disability (ulnar claw) was treated with polychemotherapy for twelve months and declared cured. Two years later, she presented to our department with painful papulonodular lesions (Figs. 1a and 1b) of abrupt onset in a febrile context, with ocular redness, lacrimation, and altered general condition. These firm, sensitive papulonodular lesions of variable size, embedded in the dermis, predominated on the trunk and face, and coexisted with sometimes hyperpigmented, sometimes hypopigmented macules of variable shape and size, with blurred borders and a smooth, non-scaly surface. A standard laboratory work-up was requested yet returned normal. A nodular lesion was biopsied, yet histological examination was not performed due to insufficient technical resources in our context. The diagnosis of type II leprous reaction (erythema nodosum leprosum) was made on the basis of the patient’s history of leprosy and the clinical picture. Treatment consisted of prednisolone 1 mg/kg/day corticosteroid therapy combined with clofazimine 300 mg/j inpatient and adjuvant therapy. An ophthalmology consultation for the management of iridocyclitis was conducted. The evolution was favorable, marked by an improvement in general condition, apyrexia, early regression, and progressive subsidence of the lesions after ten days, justifying a gradual reduction in corticosteroid therapy and, for clofazimine, a reduction of 100 mg every twelve weeks. After six months, outpatient follow-up was marked by clinical improvement, with progressive subsidence of the papulonodular lesions. Regular monthly monitoring of weight, blood pressure, blood glucose, creatinine, and blood ionogram revealed no abnormalities. After one year of treatment, all papulonodular lesions had completely subsided (Figs. 2a and 2b). No recurrence was observed after a two-year follow-up.
DISCUSSION
Erythema nodosum leprosum (ENL) is a serious immune-mediated, multi-systemic complication of lepromatous and borderline leprosy. It causes high morbidity and mortality, and usually requires urgent medical attention [4]. Lesions are dermohypodermal inflammatory nodules of varying size. In contrast to classic erythema nodosum, generally confined to the declivities, ENL is often diffuse (trunk, upper limbs, face). The lesions are smaller and accompanied by fever. They tend to recur in the same location. Other reactive equivalents may be encountered: iridocyclitis, arthritis, orchiepididymitis, and glomerulonephritis. Oral corticosteroids are the first-line treatment. Immunosuppressants (methotrexate, azathioprine, cyclosporine), anti-TNF-alpha drugs (infliximab, etanercept), or pentoxyphiline are possible alternatives [5]. Several authors have reported a recurrence of ENL [6,7], unlike our case, which was the first episode occurring two years after treatment with multidrug leprosy therapy. We did not observe a recurrence of ENL in our patient two years after the end of treatment, yet some authors have reported a variable delay in the onset of recurrence, ranging from eighteen months to two years [6,8]. In our patient, progression was favorable with prednisolone and clofazimine, although a study in India [7] reported that the rate of ENL recurrence was lower with thalidomide than with clofazimine combination therapy. Most ENL patients respond well to these treatments, while those refractory to conventional therapies may suffer severe morbidity or mortality [9]. However, therapeutic options have expanded well beyond thalidomide and steroids [10].
CONCLUSION
The diagnosis and treatment of acute erythema nodosum leprosum is particularly important in order to avoid numerous serious and disabling consequences. The particularity of our case was its early management and favorable evolution with no recurrence after two years.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. SPILF, CMIT, SFMTSI, SMV. Lèpre. Dans:ePILLY Trop [En ligne]. 3e édition web. Paris:Alinéa Plus Ed;2022. [Citéle 10/04/2023]. Disponible:https://www.infectiologie.com/fr/pillytrop.html
2. Walker SL, Balagon M, Darlong J, Doni SN, Hagge DA, Halwai V, et al. ENLIST 1:An international multi-centre cross-sectional study of the clinical features of erythema nodosum leprosum. PLoS Negl Trop Dis. 2015;9:e0004065.
3. Walker SL, Lebas E, Doni SN, Lockwood DNJ, Lambert SM. The Mortality associated with erythema nodosum leprosum in ethiopia:A retrospective hospital-based study. PLoS Negl Trop Dis. 2014;8:e2690.
4. Negera E, Tilahun M, Bobosha K, Lambert SM, Walker SL, Spencer J, et al. The effects of prednisolone treatment on serological responses and lipid profiles in Ethiopian leprosy patients with erythema nodosum leprosum reactions. PLoS Negl Trop Dis. 2018;12:e0007035.
5. Park L, Wallace CE, Vasile G, Buckley C. A case of lepromatous leprosy with erythema nodosum leprosum. Cureus. 2023;15:e33846.
6. Lastória JC, Almeida TSC, Putinatti MSMA, Padovani CR. Effectiveness of the retreatment of patients with multibacillary leprosy and episodes of erythema nodosum leprosum and/or persistent neuritis:A single-center experience. An Bras Dermatol. 2018;93:181-4.
7. P K Mishra SR, Samal R, Behera B. Thalidomide and steroid in the management of erythema nodosum leprosum. Indian J Pharmacol. 2022;54:177-82.
8. Tabassum S, Zia M, Khoja AA, David J, Iqbal M, Junaid M. Lepra reactions:A study of 130 cases from Pakistan. J Pak Med Assoc. 2021;71:2317-20.
9. Zhu J, Yang D, Shi C, Jing Z. Therapeutic dilemma of refractory erythema nodosum leprosum. Am J Trop Med Hyg. 2017;96:1362-4.
10. Bhat RM, Vaidya TP. What is new in the pathogenesis and management of erythema nodosum leprosum. Indian Dermatol Online J. 2020;11:482-92.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (contact@odermatol.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0009-0003-6095-4957 http://orcid.org/0009-0003-6095-4957 http://orcid.org/0000-0001-9843-0225 http://orcid.org/0000-0001-9843-0225 |




Comments are closed.