Androgenetic alopecia and metabolic syndrome: A cross-sectional study in Northeast India
Suman Gupta 1, Bhavya Valsalan2, Th. Bijayanti Devi2, Ronibala Soraisham3
1, Bhavya Valsalan2, Th. Bijayanti Devi2, Ronibala Soraisham3
1Skin Innovation, 2nd Floor, JR Commercial Building, Sai Nath More, Shivmandir, Siliguri, West Bengal, India, 2Regional Institute of Medical Sciences, Imphal, Manipur, India, 3Skin and STD Clinic, Imphal West, Manipur Health Services, Manipur, India
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Androgenetic alopecia (AGA) is a multifactorial progressive disorder due to excessive response to androgens. Its association with metabolic syndrome still has conflicting results.
Aim: The aim was to assess the association between androgenetic and metabolic syndrome and to study the clinico-epidemiological profile of AGA in Northeast India.
Materials and Methods: A hospital-based, cross-sectional study was conducted on 150 patients with AGA within the age group of 20–65 years. The degree of hair loss was assessed and classified according to the Hamilton and Norwood classification in males and the Ludwig classification in females. The diagnosis of metabolic syndrome (MetS) was based on criteria defined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III). Analysis was performed with IBM SPSS Statistics 21. The chi-squared test was used to assess the associations. The unpaired t-test was used to compare continuous variables. A p value < 0.05 was considered statistically significant.
Results: The prevalence of MetS in the study was 36% (54 patients). HDL was significantly (p = 0.03) lower among MetS cases than those without MetS. The components of MetS such as high WC, low-level HDL, and hypertension were significantly (p < 0.05) associated with cases of AGA with MetS. Hypertension was present among all males of grades VI and VII and all females of grade III.
Conclusion: AGA could be considered a predictor of metabolic syndrome. Patients with an early onset and higher grades of AGA should be routinely screened for MetS, which will help in preventing long-term complications such as cardiovascular diseases.
Key words: Androgenetic alopecia, Metabolic syndrome, Cardiovascular diseases
INTRODUCTION
Androgenetic alopecia (AGA) is a multifactorial disorder with progressive hair loss in specific patterns depending on circulating androgens in genetically predisposed individuals. It is characterized by stepwise miniaturization of the hair follicle, resulting from an alteration in the hair cycle dynamics, leading to vellus transformation of terminal hair follicle. Metabolic syndrome (MetS) is a group of metabolic disorders such as glucose intolerance, insulin resistance (IR), central obesity, dyslipidemia, and hypertension associated with increased risk of cardiovascular disease [1].
A previously reported association between AGA and chronic diseases, including hypertension, abnormal serum lipid profiles, obesity, insulin resistance, and cardiovascular disease (CVD) remains poorly understood [2]. Corroborating the association between metabolic syndrome and AGA may provide another clue to the clinical signs and symptoms related to both diseases [1]. Therefore, the present study was conducted to assess the association between AGA and metabolic syndrome and to study the clinico-epidemiological profile of AGA in Northeast India.
MATERIALS AND METHODS
A cross-sectional study was conducted on 150 patients with AGA attending the outpatient department of dermatology at the Regional Institute of Medical Sciences in Imphal, Manipur, for 24 months from September 2017 to August 2019. All patients presenting with AGA within the age group of 20–65 years were included, and those suffering from other types of alopecia and on glucocorticoid treatment within the previous six months were excluded. Proforma was filled and general physical examination and relevant systemic and clinical examinations were performed after obtaining informed consent. The degree of hair loss was assessed and classified according to the Hamilton and Norwood classification in males and the Ludwig classification in females. The diagnosis of MetS was based on criteria defined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III). Subjects were required to have three or more of the following: i) waist circumference (WC) > 90 cm in men and > 80 cm in women, ii) serum triglycerides (TG) ≥ 150mg/dL, (iii) high-density lipoprotein (HDL) ≤ 40 mg/dL, iv) fasting blood glucose ≥ 110 mg/dL, v) blood pressure ≥ 130/85 mmHg.
Analysis was done with IBM SPSS Statistics 21 (IBM Corp. 1995, 2012). Descriptive statistics such as frequencies, percentages, means with standard deviations, and medians were used. The chi-squared test was used to assess the associations. The unpaired t-test was used to compare continuous variables. A p value < 0.05 was considered statistically significant.
Ethics Statement
Ethical approval was obtained from the institute ethics committee.
RESULTS
The study included 150 patients. More than one-third of the cases were between 31–40 years of age (44%), followed by 20–30 (35.3%), 41–50 (12%), and 51–65 (8.7%) years, with a mean age of 32.92 ± 7.98. More than half of the cases were males (65.3%).
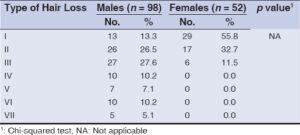
Grade III was the most common (27.6%), and grade VIII was the least common type of hair loss (5.1%) among the males (Hamilton Norwood classification), and grade I (55.8%) was the most common and grade III was least among the females (Ludwig classification) (Table 1).
A family history for AGA was the most common (91 cases; 60.7%) family history noted in patients with AGA, followed by hypertension in 53 patients (35.3%) and dyslipidemia in 39 (26%).
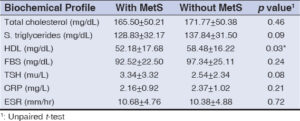
The prevalence of MetS in the study was 36% (54 patients). HDL was significantly (p = 0.03) lower among MetS cases than those without MetS. However, there was no significant (p > 0.05) difference in other biochemical parameters between AGA cases with and those without MetS (Table 2).
The components of MetS such as high WC, low-level HDL, and hypertension were significantly (p < 0.05) associated with cases of AGA with MetS. None of the other components of MetS were significantly (p > 0.05) associated with AGA (Table 3).
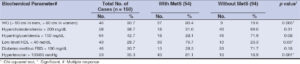 |
Table 3: Components of MetS in patients of AGA with and without metabolic syndrome (National Cholesterol Education Program: Adult Treatment Panel III). |
There was no significant (p > 0.05) association of AGA grades with the prevalence of MetS among both males and females.
When we compared the prevalence of the components of metabolic syndrome and other associated systemic diseases in the men and women with AGA grades, hypertension was present among all men of grades VI and VII and all women of grade III (Table 4).
DISCUSSION
In androgenetic alopecia, there is androgen dependent conversion of the terminal scalp hairs into miniaturized vellus hairs [3]. It is usually observed on the vertex and fronto-temporal area in men and central thinning of the crown with preserved frontal hairline seen in women. Increased 5 alpha-reductase activity with dihydrotestosterone (DHT) levels are implicated in the pathogenesis [4,5]. Hyperinsulinemia found in AGA patients favors vasoconstriction resulting in nutritional deficit in scalp hair follicles which is an important component of MetS also [6].
In the present study, the majority of AGA cases was between 31 and 40 (44%) years of age, with a mean age of 32.92 ± 7.98 years. This was in contrast to a study conducted by Wang et al. [7], in which the majority of the AGA patients belonged to the age group of 40 to 49 years (38.66%), followed by 20 to 29 years, and the mean age of presentation was 42.6 years (SD = 8.03 years).
In our study, more than half of the cases were males 98 (65.3%), compared to a study conducted by Devi et al. [8], in which 53.33% were males. In the present study, grade II hair loss of the Hamilton Norwood scale was the most common type of hair loss (28.7%). Grade I was the second most common type of hair loss (28%) and grade VII was the least common type of hair loss (3.3%). This study was in conformity with a study done by Wang et al. [7] and Shankar et al. [9], who also found grade II AGA to be the commonest type. In a study done by Batra et al. [10], more severe grades III and IV were the most common types (32% each).
In the present study, when each component of metabolic syndrome was considered individually, statistical association (p < 0.05) was identified for waist circumference, blood pressure, and HDL. Associations for the other components (TG, blood glucose, total cholesterol) were not statistically significant. Waist circumference was increased in 37 (80.4%) cases of AGA with MetS and 9 (9.6%) without MetS. This was in agreement with a study done by Devi et al. [8], who found that a high prevalence of abdominal obesity was in 25 (33.3%) cases of AGA and 11 (14.6%) controls with a significant p value (0.007). Similar findings were seen in studies done by Banger et al. [11] and Acibucu et al. [12].
In this study, hypertension was present in 81.1% of AGA cases with MetS and 18.9% of AGA without MetS with significant (p = 0.001) association. This was in conformity with studies done by Devi et al. [8] and Banger et al. [11]. The androgen-mediated receptors in the arterial wall endothelium and high serum androgen levels in AGA cases contribute to the proliferation of smooth muscle cells in vessels and increase the tendency for hypertension. Another explanation for this association is the binding of androgens to mineralocorticoid receptors, favoring increased BP or increased peripheral sensitivity to androgens despite their normal circulating levels [5].
Low-level HDL was significantly associated (p < 0.03) with 33 (76.7%) cases of AGA with MetS and 10 (23.3%) cases without MetS in our study, which was in concordance with Devi et al. [8] and Arias-Santiago et al. [13]. However, the low HDL levels may be part of the general Indian population, as found in studies by Enas et al. [14] and Sawant et al. [15].
Androgens were proven to decrease HDL levels in experimental studies. High values of TGs and low values of HDL were associated with a transition from atheroma to atherothrombosis. A negative gradient relationship between the level of HDL and the risk for moderate or severe AGA (i.e., the higher the HDL level, the lower the risk for moderate or severe AGA) was demonstrated. Therefore, investigation and control of lipid profiles in patients with AGA may be important to reduce this risk.
Regarding the family history, in the present study, we found a significant association between the presence of AGA and a family history of AGA in 91 (60.7%) cases of AGA, followed by hypertension 57 (38%), dyslipidemia 39 (26%), diabetes mellitus 37 (24.7%), thyroid disorder 19 (12.7%). This was in agreement with a study done by Bas et al. [16] who also reported that AGA was the most common family history among cases of AGA.
In this study, higher fasting blood sugar levels and hypertriglyceridemia showed an insignificant association with AGA, which was in contrast with a study by Devi et al. [8], who reported a statistical significance of high fasting blood sugar and hypertriglyceridemia with AGA.
In the present study, the prevalence of MetS in AGA patients was 36%. It was seen in 35.7% of the males and 36.5% of the females. This finding was in agreement with a study by Devi et al. [8], in which the prevalence of MS was found in 25 cases (33.3%) (18 males and 7 females). Chronic inflammation occurring in AGA with the associated increase in proinflammatory cytokines in the arterial wall may contribute to the associated cardiovascular disease [6].
In the present study, we were unable to find any significant (p > 0.05) association of AGA grades with the prevalence of MetS among both males and females.
The limitations of our study included small sample size, a cross-sectional design of the study, and lack of control groups in the study.
CONCLUSION
AGA could be considered a predictor of MetS. Patients with an early onset and higher grades of AGA should be routinely screened for MetS, which will help in preventing long-term complications such as cardiovascular diseases.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Fulop T, Tessier D, Carpentier A. The metabolic syndrome. Pathol Biol. 2006;54:375-86.
2. Sawaya ME, Price VH. Different levels of 5 alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol. 1997;109:296-300.
3. Birch MP, Lashen H, Agarwal S, Messenger AG. Female pattern hair loss, sebum excretion and the end-organ response to androgens. Br J Dermatol. 2006;154:85-9.
4. Otberg N, Finner AM, Shapiro J. Androgenetic alopecia. Endocrinol Metab Clin North Am. 2007;36:379-98.
5. Fujimoto R, Morimoto I, Morita E, Sugimoto H, Ito Y, Eto S. Androgen receptors, 5 alfa-reductase activity and androgen-dependent proliferation of vascular smooth muscle cells. J Steroid Biochem Mol Biol. 1994;50:169-74.
6. Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects:associations with obesity, insulin resistance, and endothelial dysfunction:A potential role for cytokines originating from adipose tissue?Arterioscler Thromb Vasc Biol. 1999;19:972-8.
7. Wang TL, Zhou C, Shen YW, Wang XY, Ding XL, Tian S, et al. Prevalence of androgenetic alopecia in China:A community-based study in six cities. Br J Dermatol. 2010;162:843-7.
8. Devi MM, Raju PVK, Gopal KVT, Rao TN. Study of prevalence of metabolic syndrome in androgenetic alopecia. Int J Res Dermatol. 2018;4:522-6.
9. Shanka r KD, Chakravarthi M, Shilpakar R. Male androgenetic alopecia:Population-based study in 1005 subjects. Int J Trichol. 2009;1:131-3.
10. Batra J, Khunger N, Maan KK. A study of the association of premature androgenetic alopecia with metabolic syndrome and coronary artery disease. Int J Res Dermatol. 2017;3:523-6.
11. Gopinath H, Upadya GM. Metabolic syndrome in androgenic alopecia. Indian J Dermatol Venereol Leprol. 2016;82:404-8.
12. Chakrabarty S, Hariharan R, Gowda DG, Suresh H. Association of premature androgenetic alopecia and metabolic syndrome in a young Indian population. Int J Trichol. 2014;6:50-4.
13. Agamia N. Risks for metabolic syndrome and cardiovascular diseases in both male and female patients with androgenetic alopecia. Egypt J Dermatol Venerol. 2015;35:49-55.
14. Enas EA, Mohan V, Deepa M, Farooq S, Pazhoor S, Chennikkara H. The metabolic syndrome and dyslipidemia among Asian Indians:A population with high rates of diabetes and premature coronary artery disease. J Cardiometab Syndr. 2007;2:267-75.
15. Sawant AM, Shetty D, Mankeshwar R, Ashavaid TF. Prevalence of dyslipidemia in young adult Indian population. J Assoc Physicians India. 2008;56:99-102.
16. Bas Y, Seckin HY, Kalkan G, Takci Z, Citil R, Onder Y, et al. Prevalence and types of androgenetic alopecia in north Anatolian population:A community-based study. J Pak Med Assoc. 2015;65:134-41.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-2550-4681 http://orcid.org/0000-0002-2550-4681 http://orcid.org/0009-0005-9153-0975 http://orcid.org/0009-0005-9153-0975 http://orcid.org/0009-0004-5036-1872 http://orcid.org/0009-0004-5036-1872 http://orcid.org/0009-0002-8007-9136 http://orcid.org/0009-0002-8007-9136 |





Comments are closed.