Cutaneous verrucose phaeohyphomycosis due to Exophiala oligosperma in an immunocompetent host
Corazón Barrientos-Flores 1, Denisse Vázquez-González2, Luis Miguel Moreno-López3, Javier Araiza2, Brenda Nolasco-Hernández1, Leonel Fierro-Arias2, Dalia Ibarra-Morales3, Alexandro Bonifaz2
1, Denisse Vázquez-González2, Luis Miguel Moreno-López3, Javier Araiza2, Brenda Nolasco-Hernández1, Leonel Fierro-Arias2, Dalia Ibarra-Morales3, Alexandro Bonifaz2
1Infectology Service, Instituto Nacional de Cancerología, Mexico City, México, 2Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, México, 3Dermatopathology Service, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, México
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Herein, we report a case of subcutaneous infection caused by Exophiala oligosperma. A verrucous plaque was the major clinical feature. A histopathological examination revealed features of suppurative granuloma. Mycological and molecular identification revealed E. oligosperma as the etiologic agent. After eight months of treatment, the lesion showed worsening due to the use of self-medication with topical hydrocortisone. A second biopsy was performed with direct examination (KOH 10%) showing dark pigmented septate hyphae, yeast and multiple dark hyphae were highlighted in biopsy with Gomori methenamine silver (GMS) staining. We report this case to show the rise of this infection in an immunocompetent patient and the effects of steroids. The patient was treated with itraconazole and cryosurgery.
Key words: Phaeohyphomycosis, Cutaneous, Verrucose, Exophiala oligosperma, Melanized fungi, Immunocompetent host, Itraconazole, Cryosurgery, Chromoblastomycosis
INTRODUCTION
The term phaeohyphomycosis encompasses a heterogeneous group of cutaneous, subcutaneous, and systemic diseases caused by dematiaceous or pigmented fungi detected in the tissue as yeast-like bodies, pseudohyphae-like elements, or moniliform hyphae [1]. The melanized fungi are a heterogeneous group with more than 150 species and 70 genera implicated in human and animal diseases. Most of the species involved are members of the four genera: Cladophialophora, Exophiala, Alternaria, and Bipolaris. They are ubiquitous saprobes inhabiting living and dead plant material and, for the most part, residing in the soil in extreme environments on rock, on smooth inert surfaces, or in hypersaline waters [2]. In recent decades, the frequency and biodiversity of melanized fungi as a cause of human or animal infection has increased dramatically. This correlates with the increased average age, a large number of people with treatable chronic diseases, and the growing population of immunocompromised individuals, although immunocompetent individuals may also be affected by the subcutaneous route, which is the most commonly reported form of the disease [3]. Infection occurs primarily due to traumatic inoculation of a saprobic fungus into subcutaneous tissue. In immunocompetent individuals, the disease progresses slowly from an encapsulated cyst to a swelling without ulceration [4,5].
Exophiala is a genus of anamorphic fungi of the family Herpotrichiellaceae. The main species causing human disease is Exophiala dermatitidis. Isolates exhibit marked neurotropism and are rare agents of severe, life-threatening cerebral phaeohyphomycosis, primarily in Southeast Asia [6]. The species is most frequently recovered from respiratory, cutaneous, and subcutaneous sites, and occasionally from other deep infections. However, other species recently noted as recovered from clinical samples are Exophiala oligosperma and Exophiala xenobiotica [7]. We describe a case of E. oligosperma-induced soft tissue infection.
CASE REPORT
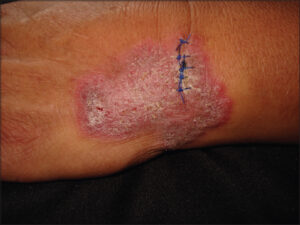
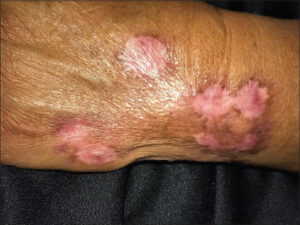
A 58-year-old male, native and resident of Tultitlan, State of Mexico (35.2 km from Mexico City), presented chronic dermatosis on his left wrist, which appeared six months earlier. The lesion slowly became verrucose and increased in size (Fig. 1). The patient had been managed at various centers with multiple medications, including oral itraconazole therapy at 100 mg/day with little relief. He had no relevant medical history and denied any contact with chemicals, arthropod bites, a family history, or contact with a person with similar injuries. He said that this hand was injured when he was playing soccer after a fall that caused a laceration. Later, he developed the swelling with a small nodular lesion on the dorsal surface of the left wrist. He had a history of traveling through several states of Mexico, including Guerrero, Morelos, and Veracruz (three humid tropical areas).
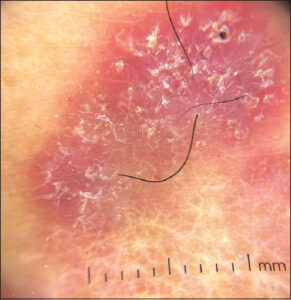
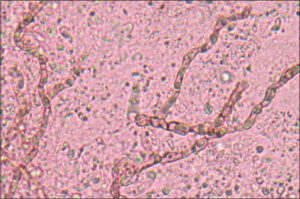
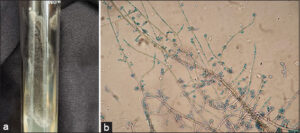
He was examined at the dermatology service. The clinical impression suggested verrucous cutaneous tuberculosis. Thorax X-ray was negative for pleuro-pulmonary and bone disease. Intradermal tuberculin reaction and sporotrichin skin test for sporotrichosis were negative. Initial dermoscopy examination found erythema, scaly areas, and black spots (hemorrhagic crusts), suggestive of chromoblastomycosis or verrucous tuberculosis. An incisional biopsy was performed on the first day that he consulted, with the histopathologic features of suppurative granuloma. Direct microscopic examinations (KOH 10%) and culture were performed on the same day of the biopsy. A black, slow-growing colony developed on Sabouraud dextrose agar (15 days, 28°C). Growth was slow first as a slightly yeast-like membranous black colony and later giving a hyphae shape (Figs. 2 and 3). After fifteen days of incubation (28°C) in Sabouraud dextrose agar, direct examination of the colony showed multiple, brown hyphae with long conidiophores that formed annelloconidia from medium length annellides, which was classified as probable Exophiala sp. Molecular identification of the isolate was done by sequencing ITS1 and ITS4 regions corresponding to Exophiala oligosperma (Figs. 4a and 4b). Chromoblastomycosis was clinically diagnosed, and treatment with terbinafine 500 mg/day was started, with clinical improvement after six months of treatment. During the eight months of treatment, the patient decided on self-medication with topical hydrocortisone. After that, the lesions showed exacerbation and worsening, and a new biopsy was performed. Direct examination (KOH 10%) revealed dark, pigmented septate hyphae, and multiple dark hyphae were highlighted with Gomori methenamine silver (GMS) staining. We decided to change the treatment for itraconazole 400 mg/day. Three cryosurgery sessions were performed, one every two months. He is currently clinically and mycologically cured and under clinical observation every three months (Fig. 5).
DISCUSSION
Dematiaceous or melanized fungi are polymorphic organisms. Due to their plasticity and adaptability to several environments, they may present a great diversity in their morphology and clinical expression [2,3].
The clinical presentation of infections caused by pigmented fungi affecting the skin and subcutaneous tissues is characterized by verrucous nodules or plaques [5]. These lesions are indistinguishable from other etiologies and mimic cases such as verrucous tuberculosis vs. verrucous sporotrichosis. Therefore, they represent a diagnostic challenge.
All conditions appearing with plaques, nodules, or ulcers are included in the tropical verrucous syndrome. It includes forms of chromoblastomycosis, sporotrichosis, lobomycosis, non-tuberculosis mycobacterial infections, leishmaniasis, tuberculosis verrucosa cutis, and noninfectious causes [8]. It is important to differentiate phaeohyphomycosis from other endemic diseases occurring in different geographic areas, especially in humid and tropical areas.
The expression of the disease depends on numerous factors, such as virulence and pathogenicity of the etiological agents and the immune system of the host against fungal infection. In patients without impaired immunity, studies have demonstrated that both innate and adaptive immune responses are necessary to clear the infection. Among the innate immune cells, macrophages and neutrophils are the first lines of defense through phagocytosis, direct elimination of pathogen, and secretion of pro-inflammatory factors. Dendritic cells (Das) are powerful antigen-presenting cells (APCs), which trigger adaptive T-cell responses against fungal infection. Patients with depletion of the CARD9 gene, which activates the protein of the same name that is necessary for the activation of T lymphocytes, show susceptibility to serious fungal infection. Chromoblastomycosis and phaeohyphomycosis represent two poles of a wide spectrum of diseases caused by distinct species of melanized fungi. Clinically, the boundaries of the spectrum are imprecise. Chromoblastomycosis and phaeohyphomycosis may be found in both immunocompetent or immunosuppressed hosts, yet phaeohyphomycosis is more frequent in immunosuppressed patients. This expression of the disease is probably related to impaired immunity. Patients with a lack of CARD 9 showed highly destructive, deep, and disseminated phaeohyphomycosis [9,10]. In most cases, samples from immunocompetent patients do not show fungal elements, and the diagnosis is obtained by growth in culture, as in our patient, who in his first biopsy only showed characteristics of a suppurative granuloma without fungus elements.
There are numerous ways in which the immune system may be affected, for instance, due to a disease such as HIV, in which the infection and depletion of CD4+T cells represent the most fundamental event in the pathogenesis of HIV-1 infection [11], or by the use of drugs such as steroids, which have effects on the immune system, for instance, hydrocortisone, which like other corticosteroids, has immunosuppressive and anti-inflammatory actions. In high concentrations, hydrocortisone attenuates the cells of defense and delays the migration of phagocytic cells to the traumatized area by reducing vasodilatation and the subsequent vascular permeability. Other effects of steroids on fungi are growth stimulating, change in morphology favoring the change from yeast to hyphae, and resistance to antimycotics, making the fungus less vulnerable to osmotic stress in the presence of the antimycotic [12,13]. With all these described effects, the steroid may aggravate the clinical course of the infection. Our patient, after the use topical of hydrocortisone, showed exacerbation and worsening of the lesions, and a new biopsy was performed.
Exophiala spp. are dematiaceous yeast-fungi acquired through accidental penetrating wounds with contaminated material. The species most frequently causing human infection are Exophiala jeanselmei, Exophiala spinifera, Exophiala dermatitidis, and other species less frequently [2,6,14]. It is of paramount importance to differentiate species of Exophiala because of their clinical, therapeutic, and epidemiological importance; they have preferred sites of infection and are associated with distinct clinical syndromes. For instance, E. jeanselmei has been reported to cause eumycetoma and, on the other hand, E. dermatitis has been associated with neurotropic infections in young immunocompetent individuals, yet these rare cases are restricted to Asia. Some species, such as the recently described Exophiala asiatica, may cause fatal disseminated cerebral phaeohyphomycosis [15].
The molecular biology method is becoming the diagnostic standard for sequencing ITS regions of the rRNA clinical isolates of Exophiala spp. Most cases of E. oligosperma cases have been reported in immunocompromised individuals. In 2003, the first English literature report of this species occurred in the Netherlands. A 62-year-old male patient with granulomatosis with polyangiitis (GPA) suffered from olecranon inflammation. The patient received amphotericin B treatment after diagnosis and was cured after ten months. Another eight cases of E. oligosperma infection were reported between 2007 and 2016 and showed global distribution, including Japan, Spain, India, and Taiwan [4,13–16]. Most of the patients had immunosuppression status. In our case, there was no evidence that the patient had impaired immune function, considering the patient had trauma before the appearance of the lesion, which we speculated could be the site of entry. There are no standardized therapies, and the treatment will be based on the extension of the disease and the immune status of the patients. This case was erroneously diagnosed as chromoblastomycosis and not as phaeohyphomycosis. However, both etiologies are covered by the same treatment. He was initially treated with subtherapeutic doses; by weight, he should have received between 300 and 400 mg/day.
Azoles are frequently used in the treatment and prevention of mycoses due to their broad-spectrum activity. Itraconazole is the most used drug for dark fungi infections. However, there is very little data available regarding the MICs [17]. In vitro studies demonstrated that posaconazole and itraconazole had the highest antifungal activity against E. jeanselmei and E. oligosperma, for which high MICs were found for caspofungin [17].
Voriconazole and posaconazole, the newly introduced third-generation drugs affecting ergosterol biosynthesis, have been used more frequently in clinical settings in recent years in some reports [18]. A Japan study summarized 32 cases of cutaneous and subcutaneous phaeohyphomycosis caused by Exophiala species and described empiric therapy with antifungals, including itraconazole, terbinafine, voriconazole, and fosravuconazole, and the treatment success rates of these monotherapies were 77% (17/22), 67% (8/12), 100% (5/5), and 50% (1/2), respectively [18]. Another study compared was YeastOne® colorimetric antifungal panels with Laboratory Standards Institute (CLSI) M38-A2 reference broth microdilution method for nine antifungals against 67 dematiaceous fungi. In this study, all azoles except fluconazole displayed low MICs, indicating these azoles may have good therapeutic effects on infections caused by dematiaceous fungi [19]. In our case, the patient was successfully treated with itraconazole for a long time. This was a case with a verrucous presentation, such as chromoblastomycosis. Cryosurgery may be an adjuvant in the treatment of resistant disease yet not only because of the possibility of lymphatic spread and to prevent a relapse.
CONCLUSION
Herein, we have reported a lesion caused by E. oligosperma. We have described that this species may produce subcutaneous infection in an induvial with normal immune status exacerbated by topical steroid management (self-medication) and with a good response to itraconazole for a long time.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
REFERENCES
1. Harris JE, Sutton DA, Rubin A, Wickes B, De Hoog GS, Kovarik C. Exophiala spinifera as a cause of cutaneous phaeohyphomycosis:Case study and review of the literature. Med Mycol. 2009;47:87-93.
2. Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23:884-928.
3. ZupančičJ, Novak BabičM, Zalar P, Gunde-Cimerman N. The black yeast Exophiala dermatitidis and other selected opportunistic human fungal pathogens spread from dishwashers to kitchens. PLoS One. 2016;11:e0148166.
4. Wu C, Shu L, Chen Z, Hu Q, Tao L, He C. Cutaneous phaeohyphomycosis of the right hand caused by Exophiala jeanselmei:A case report and literature review. Mycopathologia. 2022;187:259-69.
5. Chowdhary A, Perfect J, de Hoog GS. Black Molds and melanized yeasts pathogenic to humans. Cold Spring Harb Perspect Med. 2014;5:a019570.
6. Zeng JS, Sutton DA, Fothergill AW, Rinaldi MG, Harrak MJ, de Hoog GS. Spectrum of clinically relevant Exophiala species in the United States. J Clin Microbiol. 2007;45:3713-20.
7. Rimawi BH, Rimawi RH, Mirdamadi M, Steed LL, Marchell R, Sutton DA, et al. A case of Exophiala oligosperma successfully treated with voriconazole. Med Mycol Case Rep. 2013 2:144-7.
8. Macías P, Ordóñez J, Arenas CM, Rodríguez G. An 18-year-old man with tropical verrucous syndrome:Leishmaniasis or sporotrichosis?Biomedica. 2021;41:240-46.
9. Wu W, Zhang R, Wang X, Song Y, Li R. Subcutaneous infection with dematiaceous fungi in Card9 knockout mice reveals association of impair neutrophils and Th cell response. J Dermatol Sci. 2018;92:215-8.
10. Brito AC, Bittencourt MJS. Chromoblastomycosis:an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506.
11. Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357:1777-89.
12. Cresnar B, Zakelj-Mavric M. Aspects of the steroid response in fungi. Chem Biol Interact. 2009;178:303-9.
13. Serrano DR, Zanotti-Magalhaes EM, Magalhaes LA, Ferreira de Carvalho J. The influence of hydrocortisone on cellular defence mechanisms of Biomphalaria glabrata. Mem Inst Oswaldo Cruz. 2002;97:881-5.
14. Bossler AD, Richter SS, Chavez AJ, Vogelgesang SA, Sutton DA, Grooters AM, et al. Exophiala oligosperma causing olecranon bursitis. J Clin Microbiol. 2003;41:4779-82.
15. Venkateshwar S, Ambroise MM, Asir GJ, Mudhigeti N, Ramdas A, Authy K, et al. A rare case report of subcutaneous phaeohyphomycotic cyst caused by Exophiala oligosperma in an immunocompetent host with literature review. Mycopathologia. 2014;178:117-21.
16. Yang H, Cai Q, Gao Z, Lv G, Shen Y, Liu W, et al. Subcutaneous phaeohyphomycosis caused by Exophiala oligosperma in an immunocompetent host:Case report and literature review. Mycopathologia. 2018;183:815-20.
17. Badali H, Najafzadeh MJ, van Esbroeck M, van den Enden E, Tarazooie B, Meis JF, et al. The clinical spectrum of Exophiala jeanselmei, with a case report and in vitro antifungal susceptibility of the species. Med Mycol. 2010;48:318-27.
18. Noguchi H, Matsumoto T, Kimura U, Hiruma M, Kano R, Yaguchi T, et al. Empiric antifungal therapy in patients with cutaneous and subcutaneous phaeohyphomycosis. J Dermatol. 2022;49:564-71.
19. Zheng H, He Y, Kan S, Li D, Lv G, Shen Y, et al. In vitro susceptibility of dematiaceous fungi to nine antifungal agents determined by two different methods. Mycoses. 2019 62:384-90.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-2970-6993 http://orcid.org/0000-0002-2970-6993 http://orcid.org/0000-0003-2045-3317 http://orcid.org/0000-0003-2045-3317 |




Comments are closed.