Phototoxic drug reaction induced by hydroxychloroquine
Imane Bahbouhi , Maryem Aboudourib, Said Amal, Ouafa Hocar
, Maryem Aboudourib, Said Amal, Ouafa Hocar
Department of Dermatology and Venerology, Mohammed VI University Hospital, Av. Ibn Sina 2360, Bioscience and Health Laboratory, FMPM, Cadi Ayyad, Marrakech, Morocco
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
Sir,
Hydroxychloroquine is an anti-malarial drug often administered in systemic diseases for its immunomodulatory, anti-inflammatory, and photo-protective properties, especially in dermatomyositis and lupus erythematosus. However, antimalarial drugs have also been reported to cause photosensitivity [1].
Herein, we present a case of a phototoxic drug reaction that occurred during the treatment of cutaneous lupus erythematosus with hydroxychloroquine.
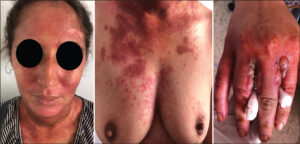
A twenty-six-year-old female treated for discoid lupus with hydroxychloroquine 200 mg twice a day and corticosteroids 40 mg a day was admitted five weeks later for a painful erythematous eruption involving sun-exposed areas. A skin examination revealed erythematous, hyperpigmented patches with bullae on sun-exposed sites, including the face, ears, posterior neck, upper back, anterior chest, forearms, and hands (Fig. 1). The eyelids, nasolabial folds, and perioral and posterior auricular regions were spared. The patient reported prolonged sun exposure several hours prior to the eruption without photoprotection.
The histopathological examination of a skin biopsy sample revealed extensive necrotic keratinocytes and dermal edema associated with an inflammatory infiltrate of lymphocytes, eosinophils, and neutrophils, consistent with a phototoxic drug eruption. Direct immunofluorescence was negative. Photopatch tests were not performed due to a lack of proper equipment at our hospital.
Hydroxychloroquine was withdrawn from the day of admission, and the eruption resolved with desquamation within several days, leaving pigmented scars. The patient did not relapse within a one-year follow-up period while maintaining corticosteroids.
Due to the high affinity of hydroxychloroquine for melanin, its highest concentration is in the skin and the eyes, which is why the most common hydroxychloroquine adverse events are ocular and cutaneous [2]. The most often described cutaneous side effects are bluish-gray hyperpigmentation, while itching, generalized pustular rash, urticaria, and erythroderma are less common [3]. Reports on hydroxychloroquine-induced photosensitivity are limited [4–6].
Photosensitive drug eruptions are cutaneous eruptions due to exposure to a medication and sunlight. Diagnosis is suspected from the clinical history, physical examination, and the temporal relationship between chemotherapy and light exposure, as well as knowledge of the classic groups of medications typically involved in such reactions, and is confirmed with clinical tests, including phototesting, photopatch testing, and histopathology [1].
Distinguishing between the two patterns of photosensitivity, photoallergic and phototoxic reactions, is based on the onset from sun exposure, clinical appearance, and histology [1].
In our patient, a rapid onset after sunlight exposure, with no prior sensitization, a clinical appearance resembling sunburn localized strictly to sun-exposed areas, with blisters and hyperpigmentation, necrotic keratinocytes in histopathology, suggested phototoxicity rather than photoallergy.
It is crucial to inform patients taking hydroxychloroquine about the potential skin-related adverse effects and recommend that they avoid direct sun exposure and use photoprotective measures.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity:Photoallergic and phototoxic reactions. Clin Dermatol. 2016;34:571-81.
2. Maghiar F, Urma M, Chiriac A, Pinteala T. Screening for ocular toxicity of hydroxychloroquine. Our Dermatol Online. 2023;14:180-3.
3. Haładyj E, Sikora M, Felis-Giemza A, Olesińska M. Antimalarials:Are they effective and safe in rheumatic diseases?Rheumatology. 2018;56:164-73.
4. van Weelden H, Bolling HH, Baart de la Faille H, van der Leun JC. Photosensitivity caused by chloroquine. Arch Dermatol. 1982;118:290.
5. Lisi P, Assalve D, Hansel K. Phototoxic and photoallergic dermatitis caused by hydroxychloroquine. Contact Dermatitis. 2004;50:255-6.
6. PesquéD, Pérez-Manich J, García-Díez I, Segura S, Ferran M, Gonzàlez-FarréM, et al. Drug-induced ultraviolet B photosensitivity due to hydroxychloroquine:The unexpected side effect. Contact Dermatitis.2023;88:168-70.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-4089-1958 http://orcid.org/0000-0002-4089-1958 |




Comments are closed.