Docetaxel-induced palmoplantar erythrodysesthesia syndrome: Dramatic presentation of a benign complication
Kavita Poonia , Purva Pande, Gurvinder Pal Thami
, Purva Pande, Gurvinder Pal Thami
Department of Dermatology, Venereology and Leprology, Government Medical College and Hospital, sector -32, Chandigarh-160012, India
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
Sir,
Docetaxel is a taxane class of cytotoxic agents used for the treatment of solid tumors [1,2]. Palmoplantar erythrodysesthesia (PE) syndrome is characterized by intense, painful erythema of the palms and soles that may progress to the formation of vesicles or bullae in a minority of patients [3,4]. We, herein, describe a case of PE after exposure to docetaxel therapy in a patient with breast malignancy.
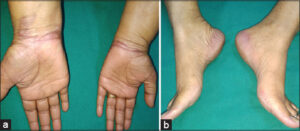
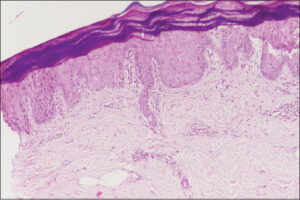
A 45-year-old female was being treated with docetaxel pulse therapy for breast cancer, given a dose of 120 mg intravenous in normal saline over a one-hour infusion. It was planned to provide four such cycles of docetaxel, and she received two cycles of treatment without any untoward effects. As per protocol, a premedication consisting of 16 mg dexamethasone and 22.75 mg pheniramine maleate was given half an hour before each cycle of therapy intravenously. After five days of the third cycle, the patient complained of a burning sensation and developed painful, erythematous, well-demarcated plaques on both hands and feet with dry, cracked, xerotic skin on the dorsal surfaces of both hands (Figs. 1a and 1b). A diagnosis of docetaxel-induced PE was considered. She underwent a skin biopsy that was suggestive of PE (Fig. 2). The discontinuation of docetaxel and treatment with 0.05% betamethasone dipropionate cream and diclofenac sodium resulted in the gradual resolution of symptoms over one week.
As per the plan of therapy, only the last (fourth) cycle of docetaxel chemotherapy was to be given, the patient was counseled about its likely recurrence and explained the benign nature of palmoplantar lesions developed in the last cycle of therapy. However, she was also reassured that an attempt to prevent such lesions should be made during chemotherapy by placing an ice pack on the palms and soles. Topical betamethasone cream and a moisturizer were also administered a week before chemotherapy. There was no complaint of a burning sensation or the development of erythematous lesions on the palms and soles except an erythematous, linear streak at the venipuncture site on the right hand (Fig. 3).
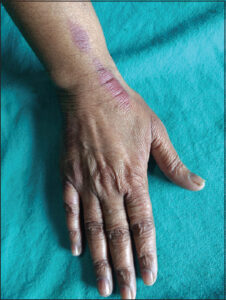 |
Figure 3: Erythematous, linear streak at the venipuncture site on the right hand after the fourth dose of docetaxel. |
The true incidence of adverse effects due to docetaxel is unknown, yet according to the literature, it ranges from 6% to 81% [1,2]. PE due to docetaxel and some other chemotherapeutics agents has been reported in the literature [2]. It has been postulated that it is due to the excretion of docetaxel through the eccrine glands resulting in a direct cytotoxic effect on epidermal cells. There are no explicit descriptions as to why this adverse effect is mainly limited to the acral parts. However, some factors differentiate the acral area from other parts of the body, such as a thick stratum corneum, temperature gradient, vibrant capillary network, rapidly proliferating cells, repeated exposure to friction/trauma, absence of sebaceous glands, and a higher number of eccrine glands, thus favoring this particular side effect on these specific sites [3,4].
PE is not a life-threatening drug reaction, hence the continuation of treatment to be weighed against its potential benefits in an individual patient. Although dose reduction is an effective method to avoid recurrence, additional preventive measures may be justified [5]. These include the avoidance of trauma, friction, exposure to heat, and regular moisturizing of the hands and feet several days before therapy. During treatment, cooling of the acral areas with ice packs has been observed to help impair circulation and decrease the excretion of chemotherapeutic drugs through the eccrine glands through vasoconstriction. Other techniques, such as the use of pyridoxine, topical steroid, vitamin E, and analgesics, are found to be effective in reducing severity [4,5].
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Patel J, Ringley JT, Moore DC. Case series of docetaxel-induced dorsal hand-foot syndrome. Ther Adv Drug Saf. 2018;9:495-8.
2. Sibaud V, Lebœuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol. 20169-10:427-43.
3. Kewan T, Alomari M, Khazaaleh S, Covut F, Olayan M. Hand-foot syndrome secondary to low-dose docetaxel in a breast cancer patient. Cureus. 2019;11:6.
4. Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes:A review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol. 2014;71:787-94.
5. Hueso L, Sanmartín O, Nagore E, Botella-Estrada R, Requena C, Llombart B, et al. Chemotherapy-induced acral erythema:A clinical and histopathologic study of 44 cases. Actas Dermosifiliogr. 2008;99:281-90.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-0450-3073 http://orcid.org/0000-0002-0450-3073 http://orcid.org/0000-0003-4821-529X http://orcid.org/0000-0003-4821-529X |




Comments are closed.