Acute generalized exanthematous pustulosis caused by sulfasalazine
Rafał Białynicki-Birula, Wiktor Cisoń, Katarzyna Krefft-Trzciniecka, Hanna Cisoń
Department of Dermatology, Venereology and Allergology, Wroclaw Medical University, Wroclaw, Poland
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
In the landscape of cutaneous adverse drug reactions (cADRs), acute generalized exanthematous pustulosis (AGEP) remains a pertinent dermatological manifestation. This research delineates a clinical presentation consistent with AGEP precipitated by the administration of sulfasalazine in a 68-year-old female. Drawing from the EuroSCAR study’s diagnostic parameters, the patient exhibited salient features such as facial erythema and a pervasive papular rash, concomitant with specific hematological perturbations, including leukocytosis and elevated hepatic enzymes. Utilizing the EuroSCAR AGEP validation score, a definitive diagnosis was ascertained, obviating the need for histological confirmation. Subsequent therapeutic interventions facilitated symptomatic resolution, leading to the patient’s discharge with a categorical advisory against future sulfasalazine exposure. This elucidation accentuates the causative potential of sulfasalazine in AGEP manifestation and underscores the imperativeness of vigilant diagnosis and timely intervention.
Key words: sulfasalazine, AGEP, Drug hypersensitivity, Drug eruptions
INTRODUCTION
Cutaneous adverse drug reactions (cADRs) encompass a spectrum of dermatological manifestations caused by pharmacotherapy. Notable classifications of cADRs include mild maculopapular exanthems (MPE), Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) [1].
AGEP has an estimated annual incidence of approximately 1 to 5 cases per million individuals [2,3]. The EuroSCAR study conducted an analysis of 97 well-documented cases of AGEP from various European countries. In more than 90% of the cases, AGEP was attributed to drug exposure. The EuroSCAR study provided insights into the diverse range of causative agents associated with AGEP. Among them, the following drugs were found to be most commonly linked to AGEP: ampicillin/amoxicillin, quinolones, hydroxychloroquine, sulfonamides, terbinafine, and diltiazem (Table 1) [4]. These medications emerged as frequently identified triggers of AGEP, as ascertained by the study findings [3,5].
CASE REPORT
A 68-year-old female with a medical history of seronegative rheumatoid arthritis and hypertension was urgently admitted to the Dermatology of Dermatology in June 2022 for prompt diagnostic evaluation and treatment of cutaneous lesions. The patient had been prescribed sulfasalazine 500 mg on May 29, 2022, which was discontinued on June 21. Subsequently, she developed facial erythema, followed by a diffuse papular rash on June 11. On June 26, the patient noticed an enlarged lymph node, and on June 28, she presented with facial edema, a fever of 39 degrees Celsius, and dysphagia.
Hospitalization records from June 22 to June 29 at the Department of Internal Medicine in Lubin indicated polymorphonuclear neutrophils > 7.000/mm³. Chest radiography revealed fibrotic changes in the left lung, while abdominal ultrasonography showed no significant abnormalities. A laryngology consultation confirmed acute inflammation of the tonsils and pharynx.
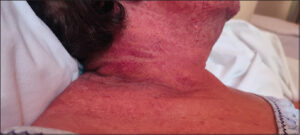
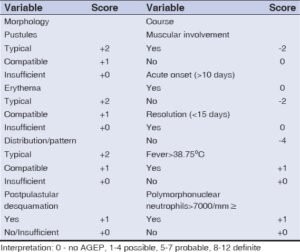
Upon admission, the patient displayed moderate contact and exhibited maculopapular rash on an erythematous background on the face, trunk, and extremities. Additionally, she presented with firm edema involving the lower limbs, extending to the knees, and lip erosions (Fig. 1). The patient’s body temperature was above 39.0 degrees Celsius. Subsequent subjective and objective evaluations prompted laboratory investigations, which revealed leukocytosis (16.72 10³/uL), anemia (erythrocytes 3.87 106 u/L), hemoglobin 11.2 g/dL, hematocrit 34.4%, thrombocytopenia (94 10³u/L), elevated neutrophils (7.26 10³/uL), and immature granulocytes (0.71 10³/uL), as well as erythroblasts (0.1 10³/uL). Furthermore, decreased levels of albumin (2.5 g/dL), potassium (2.8 mmol/l), total protein (4.7 g/dL), HDL cholesterol (29 mg/dL), and elevated levels of glucose (126 mg/dL), triglycerides (177 mg/dL), total bilirubin (1.7 mg/dL), and C-reactive protein (CRP) (17.5 mg/L) were observed. Elevated activity of liver enzymes was noted, including alanine aminotransferase (ALT) (68 U/L), aspartate aminotransferase (AST) (58 U/L), and gamma-glutamyl transferase (GGTP) (188 U/L). In the AGEP validation score of the EuroSCAR study group (Table 2), the patient received 9 points (Table 3), which is a definite diagnosis of AGEP and, that was the reason why a biopsy was not taken. An internal medicine consultation revealed no significant abnormalities. Electrocardiography (EKG) exhibited no anomalies, and the patient was advised to undergo twice-daily monitoring of the respiratory rate, receive antibiotic therapy, and maintain adequate hydration. A neurology consultation was recommended in case of clinical deterioration. Intravenous potassium supplementation was prescribed due to hypokalemia.
On the third day of hospitalization, the patient’s body temperature normalized. Laboratory tests demonstrated leukocyte count within the normal range (9.26 10³/uL), persistent anemia (erythrocytes 3.81 106 u/L, hemoglobin 10.9 g/dL, hematocrit 34.3%), and potassium within the normal range (3.6 mmol/L). Positive hepatitis B surface antibody (anti-HBs) levels (> 1000.0 mlU/ml) were detected, while hepatitis B surface antigen (HBsAg), Epstein–Barr virus IgG (EBV IgG) (12.7 U/ml), hepatitis A virus IgG antibodies, and cytomegalovirus IgG antibodies were identified. Follow-up laboratory investigations were normal.
On the ninth day of hospitalization, a psychiatric consultation was conducted, confirming the occurrence of a prior episode of delirium with a mixed etiology, in the absence of pharmacological interventions. Following the administration of intravenous and intramuscular glucocorticosteroids (dexamethasone, methylprednisolone) and phenoxymethylpenicillin antibiotic therapy, subsequent laboratory investigations revealed the absence of leukocytosis. In accordance with the laryngology consultation, a radiographic examination of the sinuses was scheduled, and the radiological report indicated a hypoplastic or partially opacified frontal sinus, while the other sinuses demonstrated normal development with air-filled spaces. The patient was discharged home in a satisfactory general condition, accompanied by an unequivocal directive to refrain from the administration of sulfasalazine.
DISCUSSION
AGEP is characterized by specific diagnostic criteria. These include the presence of pustules on an erythematous background, accompanied by desquamation upon pustule resolution. Typically, the skin manifestations initiate in interdigital regions or the facial area and rapidly disseminate to encompass the entire skin surface. In approx. 20% of cases, mucous membranes are also affected. Notably, AGEP exhibits an acute onset within ten days of exposure to the causative drug, followed by a complete remission of the skin lesions within fifteen days [6]. Additional systemic manifestations include fever (> 38°C) and peripheral neutrophilia [5,6].
In the literature, there are isolated reports on sulfasalazine being implicated in the development of AGEP [7,8].
In a study conducted by Choon et al. [9], a cohort of twenty-one patients with AGEP was examined. Among the study population, there was female predominance: 16 females and 5 males. The analysis of causative agents identified amoxicillin as the primary culprit drug, observed in ten cases. Additionally, cloxacillin was implicated in three cases, phenytoin in two cases, and individual cases were attributed to carbamazepine, allopurinol, cephalexin, ceftriaxone, celecoxib, and a herbal product. There was also one patient with AGEP after sulfasalazine inclusion.
In an investigation conducted by Vashisht et al. [10], a clinical case involving a forty-year-old female patient with a documented history of reactive arthritis was presented. The patient’s therapeutic regimen included the initiation of sulfasalazine tablets, with an initial dose of 500 mg, followed by incremental dose escalations spanning four days. Consequently, the patient manifested non-follicular pustules and erythema on the limbs. A definitive diagnosis of AGEP was conclusively established, substantiated by a flawless validation score of 12 out of 12 on the European Study of Severe Cutaneous Adverse Reaction scale.
A study conducted by Yang [11] provided evidence supporting a strong correlation between specific human leukocyte antigen (HLA) alleles and severe cADRs induced by certain drugs. Notably, the study observed a significant association between HLA-B15:02 and carbamazepine-induced Stevens–Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), HLA-B58:01 and allopurinol-induced cADRs, HLA-B59:01 and methazolamide-induced SJS/TEN, and HLA-B13:01 and sulfasalazine-induced DRESS. Unfortunately, no correlation between HLA and AGEP was observed.
CONCLUSION
While the EuroSCAR study does not explicitly identify sulfasalazine as a medication associated with a high probability of inducing AGEP, it is important to acknowledge the potential occurrence of AGEP in relation to sulfasalazine use. Patients should be informed about the plausible risks and advised to seek consultation with healthcare practitioners regarding any concerns or inquiries concerning the administration of sulfasalazine or other pharmaceutical agents.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Jinwoo L, Alyson E, Kanade S. Acute generalized exanthematous pustulosis. JAMA Dermatol. 2021;157:589.
2. Bhat YJ, Akhtar S, Muzaffar A, Iffat H, Rohi W. Etiopathological and clinical study of acute generalized exanthematous pustulosis:Experience from a tertiary care hospital in north India. Indian Dermatol Online J. 2020;11:391-7.
3. Ishikawa M, Mori T, Hanami Y, Yamamoto T. Childhood acute generalized exanthematous pustulosis. Our Dermatol Online. 2019;10:396-7.
4. Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP). Results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-96.
5. Adithyan P, Raghavendra BN, Kumar PA. Case series of a varied spectrum of drug reactions. Our Dermatol Online. 2023;14:416-9.
6. Kouotou EA, Maff o N, Degboe B, Mendouga Menye CR, Défo D, Kouassi A et. al. Acute generalized exanthematous pustulosis due to Phloroglucinol (Spasfon®):A case at the Teaching Hospital of Yaounde, Cameroon. Our Dermatol Online. 2021;14:126-10.
7. Bhat YJ, Akhtar S, Ahmad M, Hassan I, Wani R. Etiopathological and clinical study of acute generalized exanthematous pustulosis:Experience from a Tertiary Care Hospital in North India. Indian Dermatol Online J. 2020;11:391-7.
8. De A, Das S, Sarda A, Pal D, Biswas P. Acute generalised exanthematous pustulosis:an update. Indian J Dermatol. 2018;63:22-9.
9. Choon SE, Der YS, Lai NLJ, Yu SEE, Yap XL, Nalini NM. Clinical characteristics, culprit drugs and outcome of patients with acute generalised exanthematous pustulosis seen in Hospital Sultanah Aminah, Johor Bahru. Med J Malaysia. 2018;73:220-5.
10. Vashisht D, Kamboj P, Madakshira MG, Sinha P, Hegde A, Sharma J. Acute generalized exanthematous pustulosis induced by sulfasalazine:Uncommon presentation of a common culprit. Indian J Rheumat. 2022;17:435-7.
11. Yang F, Yang Y, Zhu Q, Chen SA, Fu X, Yan S, et al. Research on susceptible genes and immunological pathogenesis of cutaneous adverse drug reactions in Chinese Hans. J Investig Dermatol Symp Proc. 2015;17:29-31.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-2603-4220 http://orcid.org/0000-0002-2603-4220 http://orcid.org/0000-0003-3901-8876 http://orcid.org/0000-0003-3901-8876 |






Comments are closed.