Atopic dermatitis associated with tropical endemic limbo-conjunctivitis: Epidemiology, clinical phenotypes, and allergological investigations
Boubacar Ahy Diatta 1, Chaïma Ben Amara1, Joseph Matar Mass Ndiaye2, Patrice Mendy1, Pie Nibirantije1, Ndiague Fall1, Coumba Ndiaye1, Mame Tené Ndiaye1, Saer Diadié1, Maodo Ndiaye1, Moussa Diallo1, Fatimata Ly1, Paule Aïda Ndoye Roth2, Suzanne Oumou Niang1
1, Chaïma Ben Amara1, Joseph Matar Mass Ndiaye2, Patrice Mendy1, Pie Nibirantije1, Ndiague Fall1, Coumba Ndiaye1, Mame Tené Ndiaye1, Saer Diadié1, Maodo Ndiaye1, Moussa Diallo1, Fatimata Ly1, Paule Aïda Ndoye Roth2, Suzanne Oumou Niang1
1Department of Dermatology, Cheikh Anta Diop University of Dakar, Senegal, 2Department of Ophthalmology, Cheikh Anta Diop University of Dakar, Senegal
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Atopic dermatitis (AD) is on the increase worldwide. In Africa, 40% of cases are associated with tropical endemic limbo-conjunctivitis (TELC). Its predominance in early childhood has a major impact on the quality of life of children and their parents, leading to absenteeism from school and work. The aim of this study was to assess the particularities of AD associated with TELC in Dakar.
Materials and Methods: This was a cross-sectional, multicenter study with prospective data collection over a six-month period, conducted at dermatology and ophthalmology departments in Dakar. All patients treated for atopic dermatitis with or without TELC were included in the study. Data entry and analysis were performed with SPSS 18.
Results: From the study, 97 cases of atopic dermatitis were identified. Among these, 49 had AD associated with TELC. The sex ratio was 1.18 (36 boys and 13 girls). The mean age of patients was ten years. The age range between 5 and 10 years was more represented. There was atopy equivalent to allergic rhinitis in 44 cases, asthma in 16 cases, allergic conjunctivitis in 14 cases, and food allergy in 20 cases. 31 cases of AD were mild, 16 moderate, and 2 severe according to SCORAD. Patients were in stage I in 26 cases and in stage II in 13 cases according to Diallo’s TECL classification. All patients were managed by dermatologists and ophthalmologists. Aeroallergen prick tests were performed in thirty cases. Tests were positive for house dust mites in 23 cases (92%), animal dander in 11 cases (44%), molds in 7 cases (28%), and pollens in 2 cases (8%). Dermatophagoides pteronyssinus and farinae were the most common aeroallergens. Patch tests were positive for potassium dichromate in 4 cases, cobalt in 4 cases, nickel in 3 cases, and lanolin in 4 cases.
Conclusion: AD associated with TECL remains common in tropical environments. They share common aggravating environmental factors, notably hypersensitivity to aeroallergens and a predominance in early childhood. The major impact on functional prognosis makes therapeutic education and multidisciplinary management of patients essential.
Key words: Atopic dermatitis, Tropical limbo-conjunctivitis, Dakar
INTRODUCTION
Atopic dermatitis (AD) is a chronic, pruritic inflammatory dermatosis that progresses through flare-ups and remissions. It is a multifactorial disease combining genetic and environmental factors [1]. It is often associated with other atopic equivalents such as allergic rhinitis, allergic conjunctivitis, or asthma [2,3]. It affects at least 230 million people worldwide, and its prevalence rose in 30 years from 5% to 25% [4,5]. Several studies have shown an association between AD and tropical endemic limbo-conjunctivitis, with an estimated prevalence of 40% [6]. TECL is an endemic limbo-conjunctivitis that occurs mainly in children in tropical environments. The frequency of TECL reported in Africa varies between 2.8% and 90% [7,8]. Its association with AD may be linked to common environmental factors: tropical, sunny climate, photosensitivity, and exposure to aeroallergens. Allergological investigation helps to identify trigger factors and establish avoidance measures for therapeutic education. Little work has been done in Africa on the epidemiology of patients with AD associated with TECL. The aim of this study was to determine the epidemiological, clinical, and etiological features of patients with atopic dermatitis associated with tropical endemic limbo-conjunctivitis.
MATERIALS AND METHODS
This was a descriptive, multicenter, cross-sectional study conducted over a six-month period (April 1 to September 30, 2022) at the dermatology departments of Aristide Le Dantec Hospital, Hygiena Social Institute, Albert Royer Children Hospital, and the Ophthalmology Department of Aristide Le Dantec Hospital. Our study population consisted of patients seen in consultation at these different departments. All patients presenting with atopic dermatitis associated with TECL were included in the study. The diagnosis of AD was based on the existence of pruritus and eczematous lesions on the folds, extension surfaces of the limbs, and convex areas in children, or of cutaneous xerosis and minor signs of atopy (periorbital hyperpigmentation, Dennie–Morgan double fold, keratosis pilaris, palmoplantar hyperlinearity, follicular eczematitis, achromic eczematitis). The United Kingdom Working Party diagnostic criteria were employed [9]. The diagnosis of TECL and classification into four clinical stages according to Diallo’s classification [7] were performed by experienced ophthalmologists. The patients were asked to complete a questionnaire and undergo a full clinical examination. Allergological tests included patch tests using the European standard battery or personal products, respiratory or food prick tests and specific IgE assays for aeroallergens or food allergens. All patients received therapeutic education. Data entry and analysis were performed with Excel and SPSS 18. Chi-square and Fisher tests were used according to their conditions of applicability, with a significance level of p < 0.05.
RESULTS
We identified 49 cases of atopic dermatitis associated with TCEL out of 97 patients presenting with atopic dermatitis during our study period, giving a hospital prevalence of 50.5%. Thirty-six cases (73.4%) were followed up at the dermatology department and 13 cases (26.5%) at the ophthalmology department. The sex ratio was 1.18 (36 boys and 13 girls). The mean age of the patients was 10 years, with extremes ranging from 3 to 20 years. The age range between 5 and 10 years was more represented. Fig. 1 illustrates the distribution of patients according to age of onset of AD and TECL. There was an atopy equivalent in the form of allergic rhinitis in 44 cases, asthma in 16 cases, allergic conjunctivitis in 14 cases, and food allergy in 20 cases. Tropical endemic limbo-conjunctivitis was present in at least one family member in 32 cases (32%).
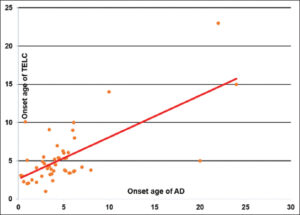 |
Figure 1: Distribution according to the onset age of AD and tropical endemic limbo-conjunctivitis (TECL). |
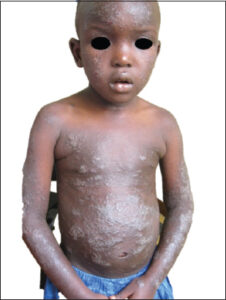
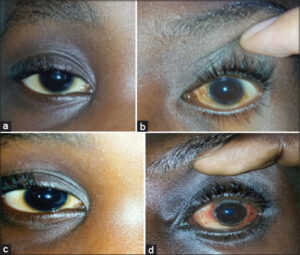
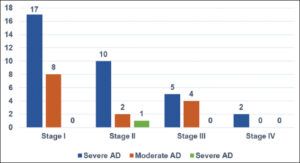
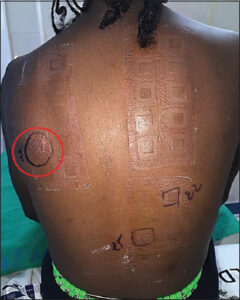
The average consultation time was 7.2 months, with extremes ranging from one week to fourteen years. According to SCORAD, atopic dermatitis was mild in 31 cases, moderate in 16 cases, and severe (Fig. 2) in 2 cases. According to Diallo’s TECL classification, patients were stage I in 26 cases, stage II in 13 cases, stage III in 9 cases, and stage IV in 2 cases (Fig. 3). Fig. 4 illustrates the distribution of cases according to the severity of AD and TECL. All patients were mutually managed at the dermatology and ophthalmology departments. Respiratory prick tests were positive for house dust mites in 23 cases (92%), animal dander in 11 cases (44%), molds in 7 cases (28%), and pollens in 2 cases (8%). Table 1 illustrates sensitization to respiratory allergens. Patch tests showed sensitization to metals: potassium dichromate, cobalt, and nickel (Fig. 5). Table 2 shows patch test sensitizations. Food-specific IgE tests were conducted in 4 cases (4%) and were positive for shrimps and eggs.
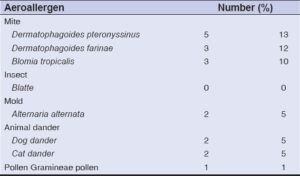 |
Table 1: Positivity of skin tests to aeroallergens. |
 |
Table 2: Tests positive for European standard battery allergens. |
A concordance between the European standard battery and the products reported by the patient reinforced the relevance in one case for nickel. The relevance of the tests was current in 18 cases and long-standing in 4 cases.
DISCUSSION
We report 49 cases of atopic dermatitis associated with TECL. Half of the patients who consulted us for atopic dermatitis presented with TECL during our study period. The prevalence varies in Africa, with some authors estimating it at between 3.4% and 83%. [8,10]. We noted a predominance of males among patients with AD and TECL. This data is consistent with previous studies [7,11] Atopic dermatitis has always been considered a disease of early childhood, classically beginning before the age of two years and generally subsiding in adolescence [12]. We noted a predominance of DA and TECL symptoms in children under seven years of age. Tropical limbo-conjunctivitis is a pathology that classically affects children before adolescence [1,13]. The average age of the patients in our series was less than ten years [14]. It was described by Diallo in 1971, mainly affecting children between 3 and 16 years of age and is often equated with vernal keratoconjunctivitis. However, it differs from vernal keratoconjunctivitis in that its limbic involvement is more marked, and its corneal complications are the main cause of its severity [15,16]. These same factors may also be responsible for atopic dermatitis flare-ups. In fact, AD and TECL share the same environmental factors that cause flare-ups. In our series, tests were positive for house dust mites (92%), animal dander (44%), molds (28%), and pollens (8%). Our results were identical to those found in the literature. House dust mites are the aeroallergens most frequently associated with AD [17].
A clear predominance of aeroallergens (house dust mites, cockroach, dog and cat, and grass pollen) was also more prevalent in children followed for TECL in Togo [18]. Metal sensitization noted in our study is also observed in previous studies in the literature [17]. Nickel is a frequent contact allergen in the face. Sensitization may be direct, via cosmetic products or ophthalmic topicals, or indirect, via the handling of contact allergens [19].
CONCLUSION
Atopic dermatitis is frequently associated with TECL in tropical environments. They share common aggravating environmental factors, notably hypersensitivity to aeroallergens and a predominance in early childhood. The major impact on patients’ functional prognosis and quality of life makes therapeutic education and multidisciplinary management essential.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Fishbein AB, Silverberg JI, Wilson EJ, Ong PY. Update on atopic dermatitis:Diagnosis, severity assessment, and treatment selection. J Allergy Clin Immunol Pract. 2020;8:91-101.
2. David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis:pathophysiology. Adv Exp Med Biol. 2017;1027:21 37.
3. Yang L, Fu J, Zhou Y. Research progress in atopic March. Front Immunol. 2020;11:1907.
4. Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, et al. Epidemiology of atopic dermatitis in adults:Results from an international survey. Allergy. 2018;73:1284 93.
5. Bylund S, Kobyletzki LB, Svalstedt M, Svensson Å. Prevalence and incidence of atopic dermatitis:A systematic review. Acta Derm Venereol. 2020;100:160.
6. Técléssou JN, Mouhari-Toure A, Akakpo S, Bayaki S, Boukari OB, ElégbédéYM, et al. [Risk factors and allergic manifestations associated with atopic dermatitis in Lomé(Togo):A multicenter study of 476 children aged 0-15 years]. Med Sante Trop. 2016;26:88-91.
7. Diallo J. La limbo-conjonctivite endémique des tropiques. Rév Int Trach Pathol Ocul Trop Subtrop. 1976;34:71-80.
8. Epée E, Koki G, Dohvoma VA, Kenne C, Biangoup NP, Tocke O, et al. Epidemiological and clinical aspects of tropical endemic limbo-conjunctivitis in students in Yaoundé. J Fr Ophtalmol. 2016;39:744 9.
9. Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. Working Party’s Diagnostic Criteria for atopic dermatitis. III Independent hospital validation. Br J Dermatol. 1994;131:406-16.
10. Brémond-Gignac D, Nischal KK, Mortemousque B, Gajdosova E, Granet DB, Chiambaretta F. Atopic keratoconjunctivitis in children:Clinical features and diagnosis. Ophthalmology. 2016;123:435 7.
11. Ahogo KC, Kouassi YI, Gbery IP, Azagoh KR, Yeboua KI, Kouassi KA, et al. Atopic dermatitis in children:Epidemiological and clinical aspects in Côte d’Ivoire. Our Dermatol Online. 2017;8:25-7.
12. Lee HH, Patel KR, Singam V, Rastogi S, Silverberg JI. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. JAAD. 2019;80:1526-32.
13. Mvogo SRE, Dohvoma VA, Tsimi CM, Ndongo JAJ, Mbia DAZ, Komatchou DS, et al. [Measurement of lacrimal secretion in patients with tropical endemic limbo-conjunctivitis:A case control study]. J Fr Ophtalmol. 2022;45:903-7.
14. Ohshima Y, Yamada A, Hiraoka M, Katamura K, Ito S, Hirao T et al. Early sensitization to house dust mite is a major risk factor for subsequent development of bronchial asthma in Japanese infants with atopic dermatitis:Results of a 4-year follow-up study. Ann Allergy Asthma Immunol. 2002;89:265-70.
15. 15 – Ndoye Roth PA, Ngatcho Hemo J, Wane Khouma AM, Ba EH, Sow AS, Ka AM, et al. Intratarsal triamcinolone acetonide injection for treatment of refractory chronic tropical endemic limboconjunctivitis (TELC):Experience at Aristide-Le-Dantec university hospital in Dakar. J Fr Ophtalmol. 2012;35:266-71.
16. Everaerts MC, Doutetien C. [Chronic tropical endemic limboconjunctivitis (TELC) in southern Benin:epidemiological and meteorological data]. Rev Int Trach Pathol Ocul Trop Subtrop Sante Publique. 1993;70:199-214.
17. Tamagawa-Mineoka R, Katoh N. Atopic dermatitis:Identification and management of complicating factors. Int J Mol Sci. 2020;21:26-71.
18. Banla M, Maneh N, Vonor K, Vonor B, Nonon Saa KB, Agba A, et al. Tropical endemic limbo-conjunctivitis (TELC) and allergic management:A preliminary study in Togolese children. J Fr Ophtalmol. 2013;36:677 82.
19. Verhulst L, Person L, Zimerson E, Bruze M, Vanden Broecke K, Goossens U. Palpebral eczematous dermatitis caused by nickel in an eye pencil. Contact Dermatitis. 2014;70:247 9.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0001-8923-0790 http://orcid.org/0000-0001-8923-0790 http://orcid.org/0000-0003-4530-1686 http://orcid.org/0000-0003-4530-1686 http://orcid.org/0000-0002-9325-4784 http://orcid.org/0000-0002-9325-4784 http://orcid.org/0000-0002-8961-6973 http://orcid.org/0000-0002-8961-6973 http://orcid.org/0000-0001-9770-1118 http://orcid.org/0000-0001-9770-1118 http://orcid.org/0000-0001-5812-2770 http://orcid.org/0000-0001-5812-2770 http://orcid.org/0000-0002-8245-6372 http://orcid.org/0000-0002-8245-6372 http://orcid.org/0000-0003-4132-9315 http://orcid.org/0000-0003-4132-9315 http://orcid.org/0000-0002-7255-8471 http://orcid.org/0000-0002-7255-8471 |




Comments are closed.