Nifuroxazide and erythema pigmentosa: A side effect to be aware of
Ryme Dassouli , Zakia Douhi, Imane Kacimi, Kenza Tahri, Hanane Baybay, Sara Elloudi, Fatima Zahra Mernissi
, Zakia Douhi, Imane Kacimi, Kenza Tahri, Hanane Baybay, Sara Elloudi, Fatima Zahra Mernissi
Department of Dermatology, University Hospital Hassan II, Fes, Morocco
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
Sir,
Erythema pigmentosa fixata (EPF) is a delayed-type toxidermia. It manifests itself within forty-eight hours after the reintroduction of a drug by the appearance of a recurrent rash, leaving a residual hyperpigmentation [1]. Herein, we report the first case of erythema pigmentosa fixed to nifuroxazide.
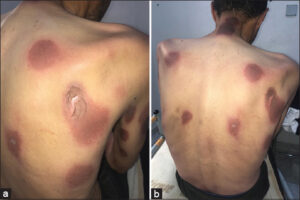
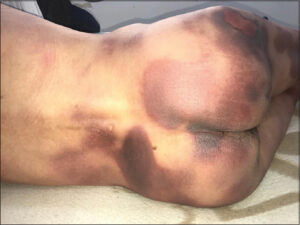
A 48-year-old male, a chronic smoker, with a history of type 1 diabetes under insulin, having four limbs amputated five years previously for ischemia of the limbs secondary to Shepherd’s disease, suffering from a functional colopathy, for which the patient often received symptomatic treatments, including nifuroxazide. A drug investigation revealed the ingestion of nifuroxazide for a digestive episode, after which the patient presented a rash composed of pruritic, erythematous macules of the trunk and limbs with a 48-hour delay, evolving into post-inflammatory hyperpigmentation (Figs. 1 and 2). A biopsy showed an interface dermatitis consisting of a predominantly lymphocytic infiltrate. A pharmaco-vigilance report accused nifuroxazide. All data allowed us to conclude the diagnosis of erythema pigmentosa fixata. The patient presented no recurrences after the eviction of the molecule.
Nifuroxazide is widely prescribed for its analgesic and anti-bacterial properties in various digestive diseases, including functional colopathy. Nifuroxazide, identified as a STAT3 inhibitor, has also proven to be effective in the treatment of certain tumor pathologies, due to its anti-tumor properties and ability to induce apoptosis [2]. Numerous adverse effects have been reported with nifuroxazide, particularly in the skin: urticaria, allergic reactions, angioedema, and anaphylactic shock.
EPF is most often benign. More rarely, it may be bullous and extend to the oral and genital mucosas. A pharmacovigilance investigation is necessary to establish the causal link between the EPF and the responsible drug. The diagnosis of EPF is clinical, characterized by the reappearance of lesions on the same site of the initial outbreak after the reintroduction of the offending drug [3].
The discontinuation of the offending drug must be formally and permanently indicated [3].
Usually, the drugs most frequently responsible for EPF are sulfonamides, non-steroidal anti-inflammatory drugs, and tetracyclines, although this frequency may vary depending on consumption habits and the emergence of new drugs [4].
It is recommended to perform patch tests on the site previously affected by EPF, and this seems to be more appropriate when several molecules are attributable [4].
Indeed, our observation is the first case of EPF induced by nifuroxazide reported in the literature.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Fayt G, Lejeune C, Arco D, Higuet S. [News Skin lesions:A case report of fixed drug eruption]. Rev Med Brux. 2017;38:439-41.
2. Luo Y, Zeng A, Fang A, Song L, Fan C, Zeng C, et al. Nifuroxazide induces apoptosis, inhibits cell migration and invasion in osteosarcoma. Invest New Drugs. 2019;37:1006-13.
3. Lakhoua G, El Aidli S, Zaïem A, Sahnoun R, Kastalli S, Hedi Loueslati M, et al. [Fixed pigmented erythema antihistamine H1:About 2 cases and review of the literature]. Therapie. 2014;69:243-4.
4. Valeyrie-Allanore L, Lebrun-Vignes B, Bensaid B, Sassolas B, Barbaub A;sous l’égide du groupe toxidermie de la Sociétéfrançaise de dermatologie (FISARD). [Fixed pigmented erythema:Epidemiology, physiopathology, clinical features, differential diagnosis and therapeutic management]. Ann Dermatol Venereol. 2015;142:701-6.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-4330-4429 http://orcid.org/0000-0003-4330-4429 http://orcid.org/0000-0003-3455-3810 http://orcid.org/0000-0003-3455-3810 http://orcid.org/0000-0002-5942-441X http://orcid.org/0000-0002-5942-441X |




Comments are closed.