Recurrent pityriasis versicolor: A short review of clinical features and antifungal and non-antifungal treatment options
Andrés Tirado-Sánchez1,2, Mariana G. Ungson-García3, Mariel Isa-Pimentel4, Leonel Fierro-Arias1, Sofía Beutelspacher1, Sandra Miranda-Mauricio5, Alexandro Bonifaz 1
1
1Dermatology Department, Hospital General de México, México city, Mexico, 2Internal Medicine Department, Hospital General de Zona 30, Instituto Mexicano del Seguro Social, México City, Mexico, 3Dermatology Service, Hospital Reginaol “Dr Valentín Gómez Farias” ISSSTE, Zapopan, Jalisco, Mexico, 4Instituto Dominicado de Dermatología y Cirugía de Piel “Dr Huberto Bougart. Santo Domingo, Dominican Republic, 5Central Laboratory, Hospital Adolfo López Mateso. ISSSTESON, Cd. Obregón, Sonora, Mexico
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Objective: We conducted a systematic review of the literature from the PubMed database from January 1, 2010, to December 31, 2021. The search criteria were “(pityriasis versicolor OR tinea versicolor) AND treatment,” with the full text available and the English language required. This review focuses on the clinical evidence supporting the efficacy of antifungal and non-antifungal treatment for pityriasis versicolor.
Background: Pityriasis versicolor is a chronic superficial mycosis caused by the Malassezia species. The condition is one of the most common infections worldwide, particularly in tropical climates. Although it is a superficial infection, recurrences are high due to the presence of Malassezia in the normal skin flora.
Summary: Topical and oral antifungal treatments effectively reduce the recurrence, leading to a lasting clinical and mycological cure. In addition to antifungal therapies, non-antifungal treatments have shown efficacy in cases of recurrent pityriasis versicolor and could be used as maintenance or preventive therapy. Due to high recurrence rates, prophylactic treatment may be necessary.
Key words: Pityriasis versicolor, Superficial mycosis, Tinea versicolor, Treatment
INTRODUCTION
Pityriasis versicolor (PV) is a superficial chronic fungal infection caused by yeasts of the Malassezia spp. genus [1,2]. These species are commensals on human skin and warm-blooded animals, such as pigs, monkeys, goats, horses, dogs, cats, and others, developing skin diseases and systemic infections in humans and animals [3].
Malassezia yeasts have been classified into at least fourteen species; eight have been isolated from human skin. The frequency varies depending on country; M. furfur is the main species in Indonesia and Brazil, M. sympodialis in Canada, and M. sympodialis and M. globosa in Argentina. The isolation of M. slooffiae and M. restricta is less frequent [1].
M. sympodialis is the predominant species in human skin, healthy or diseased, and is usually found on the trunk, while M. globosa is found in PV scales and healthy skin and M. restricta seems to be associated with pityriasis capitis [4]. M. pachydermatis, an agent of external otitis in cats and dogs, has also been considered responsible for some cases of systemic infection, mainly in premature children [1].
Malassezia-related skin diseases include head and neck dermatitis, seborrheic dermatitis, PV, and Malassezia folliculitis [5]. M. japonica, a recently described species was isolated mainly in the Japanese and Chinese populations with psoriasis, atopic dermatitis, seborrheic dermatitis, and healthy individuals [6]. Romero-Sandoval et al. [7] reported the isolation of M. japonica from patients with PV refractory to treatment. M. globose in the mycelial phase is the causative agent of typical and disseminated PV [8].
EPIDEMIOLOGY
Pityriasis versicolor has a worldwide distribution, affects all races, and is most prevalent in tropical and subtropical regions, where high humidity and temperature increase disease prevalence [9]. It may affect 40–50% of individuals from specific geographic regions (temperate climates) and ethnic groups [4]. It may occur at any age yet is more common in adolescents and young adults, with a peak incidence between the second and fourth decades of life. In most series, both sexes are equally affected, although there may be a slight male predominance depending on the series studied [1,4].
Numerous epidemiological studies have been conducted globally, with M. globosa being the principal etiological agent in regions with a temperate climate; in tropical and subtropical climate regions, the most common species are M. sympodialis, M. furfur, and M. globose [9–11].
PATHOGENESIS
The factors involved in transforming the yeast into its pathogenic mycelial form are uncertain. Endogenous and exogenous factors include genetic inheritance, congenital or acquired immunosuppression, malnutrition, oral contraceptives and corticosteroids, hyperhidrosis, endocrine disorders, elevated temperature, humidity, occlusive clothing, use of oil or moisturizers on the skin, as well as the chemical sebum composition [12].
Malassezia yeasts are lipophilic fungi with a high dependence on a lipid-rich microenvironment, and these microorganisms are part of the skin microbiome [13]. Under certain conditions, the yeasts become a pathogenic agent and produce various skin diseases, even systemic diseases. These fungi are mainly found in the infundibulum of the sebaceous glands, where lipids are widely available. It often requires peptone-rich media to grow, containing short-chain fatty acids (M. pachydermatis, M. furfur) [14,15].
The virulence factors of these yeasts, intrinsic factors of the host, and extrinsic environmental factors are involved in the pathogenesis of PV. The Malassezia cell wall represents the initial point of interaction between the host and pathogen. Its composition is strongly associated with adherence and penetration to tissues, helping it evade host defenses. The cell wall of M. furfur and M. pachydermatis is mostly galactomannans (galactose and mannose) and glucose [14,15].
Adhesion to host cells is necessary for colonization and infection. In addition to the cell wall characteristics, it also exhibits hydrophobic cell surface characteristics, which promote biofilm formation on biological surfaces and inert surfaces in approx. forty-eight hours in polyurethane catheters. This property gives it increased virulence, resistance to antifungal penetration, and drug resistance, which seems to be associated with the production of systemic infections. Numerous microorganisms generate hydrolytic enzymes that help them in their pathogenicity [13–15]. Malassezia species may produce proteinases, lipases, phospholipases, hyaluronidases, and chondroitinsulfatases, which promote the formation of pores in cell membranes, dismantling cell function, and promoting tissue invasion, with the subsequent dispersal of organisms. These lipases destroy triglycerides in the sebaceous glands and produce numerous unsaturated free fatty acids, local irritants, and immunostimulants [12–15]. Complex interaction begins once the yeasts encounter the stratum corneum and colonize it. It has been seen that the Malassezia species, under normal conditions, favor the production of transforming growth factor b1 and interleukin (IL) 10, which are powerful immunomodulatory and immunosuppressants decreasing the local response against these yeasts and facilitate the colonization of the skin [12,15].
Once the yeasts penetrate the stratum corneum, these are recognized and phagocytosed by local dendritic cells or Langerhans cells, which recognize and process mannose receptors and present to B and T cells in lymph nodes [1,7,12]. The importance of this T response is reflected in patients with HIV, in whom there is a significant proliferation of Malassezia and, in addition, the appearance of seborrheic dermatitis that is difficult to control, related to lymphopenia. It has also been seen that a specific group of patients may have an idiosyncratic response to the Malassezia species that favor a type IV hypersensitivity reaction, resulting in a TH1-type response, in addition to increased activity of metalloproteinases, which are potent inhibitors of elastic fiber synthesis, resulting in atrophic skin lesions [1,16,17].
Malassezia is able to convert tryptophan to a wide variety of indole compounds associated with some of the clinical features of PV, such as the hypopigmentation seen in some lesions. This has been linked to the induction of melanocyte apoptosis, mediated by the activation of the aryl hydrocarbon receptor, which results in the transcription of cytochrome p450 proteins and the stimulation of the caspase pathway [18]. This clinical phenomenon (hypopigmentation) is also explained by the production of azelaic acid, which inhibits the synthesis of tyrosinase, an enzyme that mediates the conversion of L-DOPA to melanin [14]. On the other hand, in ultrastructural studies, it has been observed that some lesions exhibit a decrease in the number, size, and distribution of melanosomes, which would also explain the hypochromic described above. Although indeed, the exact cause of the hyperpigmented variant is unknown, it is suspected to be due to an increase in the thickness of the epidermis, as well as a more remarkable lesional inflammatory infiltrate, which would stimulate the melanocytes to produce more pigment and culminate with an increase in the number, size, and distribution of melanosomes [14].
CLINICAL MANIFESTATION
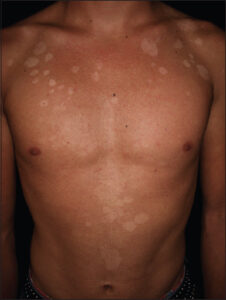
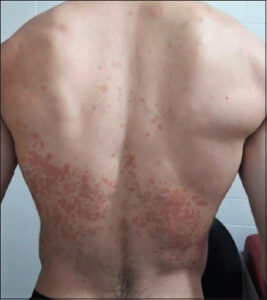
The lesions include round or oval macules, papules, or isolated plaques that may coalesce and cover large body areas separated by normal skin, leading to pigmentary changes from hypochromic macules (mainly related to the increased production of Malassezia-derived dicarboxylic acids, including azelaic acid with the competitive inhibition of tyrosinase) to erythematous or hyperchromic lesions (due to abnormally large melanosomes) [14,17]. Patches of PV have a brown or yellowish color and, if scraped with the fingernail, furfuraceous scaling is observed (Besnier’s sign or scratch sign). Zireli’s sign is characterized by scaling when the skin is stretched, and it is pathognomonic of PV [1] (Figs. 1 and 2).
DIAGNOSIS
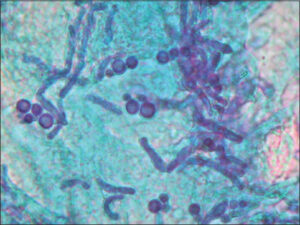
The diagnosis is based on typical clinical manifestations combined with bright yellow fluorescence under Wood’s light and direct mycological examination. The methods of lesion scraping or adhesive tape may be employed for material collection and observation under an optical microscope [5]. Potassium hydroxide (10% to 20%) with methylene blue 1% or Albert solution (toluidine blue and malachite green) is used for better visualization of fungal structures. On direct examination, yeast cells and hyphae are easily identified [19]. Vitiligo, pityriasis alba, hypochromic mycosis fungoides, and postinflammatory hypopigmentation should be considered in the differential diagnosis [20] (Fig. 3).
TREATMENT
We detected several systematic reviews focused on the different topical and systemic treatment options in the management of PV (Table 1). The four most important reviews in the search parameters focus on the efficacy of topical ketoconazole, and in cases that require systemic management, the best options are itraconazole and fluconazole [21–24]. The lesions may be recurrent or recalcitrant to antifungal treatments [21,25]. Recurrent cases include extensive or disseminated cases with atypical variants related to infectious agents such as M. japonica and elevated IgE levels [7,26].
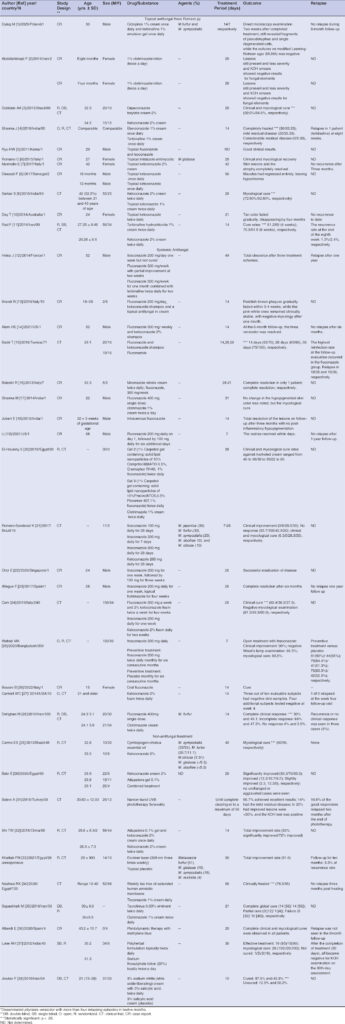 |
Table 1: Studies published between 2011 and 2021 on topical and systemic antifungal and non-antifungal treatments. |
Pityriasis versicolor may persist chronically if left untreated. Numerous topical and oral antifungal treatments effectively alleviate clinical symptoms and lead to mycological cure. First-line treatment includes topical antifungal therapy [27]. Topical azole antifungals are effective for PV, and no significant difference in the efficacy of different azoles is observed. Ketoconazole shampoo, selenium sulfide, or zinc pyrithione are recommended in hairy areas; due to the difficulty of applying the antifungal cream, these are applied to the skin in a shower and washed off after 3 to 4 minutes for 2–3 weeks [28]. Terbinafine 1% cream and ketoconazole 2% cream are adequate, similar to new antifungals such as topical fluconazole. The usual treatment time is 2 to 3 weeks [29].
Based on evidence, treatment once or twice daily for fourteen days with topical ketoconazole cream or foam and once-weekly ketoconazole shampoo may be effective for PV with long-term efficacy. Similarly, terbinafine 1% cream should be applied twice daily for seven days [21]. Keratolytic soaps, such as sulfur-salicylic acid soaps, demonstrated a 62% cure rate and had the best results with extended duration of application, yet when compared to 1% clotrimazole, the latter had better results [30].
Shi et al. [31] compared ketoconazole 1% cream as monotherapy with ketoconazole cream combined with adapalene 1% gel. Adapalene is a naphthoic acid derivative indicated for treating acne vulgaris, because it binds to retinoic acid receptors (RAR) located mainly in the skin and epidermis (RARb and RARg, respectively), inhibiting cell differentiation. This study defined the total improvement rate as negative direct microscopy and a clinical improvement of 50% four weeks after treatment. The combined treatment significantly improved (92% vs. 72%, respectively; p = 0.0009). In a study by Bakr et al. [32], the response to treatment was significantly different when the combined treatment of ketoconazole 2% cream and adapalene 0.1% gel was administered as compared to the treatments as monotherapy at seven weeks (93.3/70/83.3, respectively).
Regardless of the medication administered, the normalization of pigmentation may take several months after the completion of treatment [1,2].
RECURRENT PV
Although the infection does not represent a significant health risk to the affected individual, the psychological and social implications may be profound. The disease becomes chronic without treatment. A relapsing disease tends to recur in around 60% of the cases within a year after treatment. Spontaneous remissions are rare [3].
It is also described as recurrent, recalcitrant, or relapsing PV. The disease evolves in outbreaks, with the improvement and aggravation of the symptoms, leading to relapse. Due to several predisposing factors, relapse is a significant problem. PV may recur after incomplete antifungal treatments [2,3]. Faergemann reported a relapse rate of 60% after one year and 80% after two years of treatment [33]. Relapse probably occurs due to the presence of yeasts in the sebaceous follicles and several predisposing factors that allow the multiplication and filamentation (hyphae formation) of yeasts. During the twenty-month follow-up, relapse was observed in periods of excessive sweating caused by physical exercise or after spending time at the beach, pool, and farm. The patients also associated relapses with higher temperatures (summer) or the application of oily products to the body (moisturizers, sunscreens). The patients with PV with one to four relapsing episodes in twelve months were classified as having relapsing PV [3].
Drug resistance is another increasing problem affecting all antifungal agents [34]. Azole resistance may be primary (intrinsic) or secondary (acquired). The former is found naturally without prior (known) antifungal exposure. The latter results from a previously susceptible strain exposed to antifungals or other selective pressure and may result from altered gene expression, point mutations, or allelic variations. Both may be attributed to an increase in 1) the prophylactic use of azole drugs, 2) prolonged treatment regimens, 3) agricultural use of azole fungicides, or 4) the broad-spectrum, long-term, and low-dose use of azoles in consumer care. As an example, azole resistance has been extensively studied for Aspergillus fumigatus. It develops either during treatment at the hospital or following intensive agricultural practice [35]. The environmental route of resistance development has been reported since 2007 [35]. Malassezia azole resistance is associated mainly with mutations in the ERG11 gene, identified from clinical isolates [36].
TREATMENT OF RECURRENT PV
In recurrent, disseminated, or recalcitrant cases with topical therapy or patients who experience multiple relapses, oral itraconazole 100 mg daily for fourteen days may be a practical option [37]. Oral fluconazole is also effective and safe [38,39]. The cure rate ranged from 78% to 98% when fluconazole was started once weekly for two weeks [38]. A randomized controlled trial on adults demonstrated that a single high-dose (400 mg) fluconazole treatment may be more effective than itraconazole (65% vs. 20%, respectively); the relapse rate was 35% in the fluconazole group and 60% in the itraconazole group at the end of eight-weeks follow-up [39].
Other treatment options, including adapalene gel with ketoconazole cream [31,32], increase the success rate of topical ketoconazole cream from 72% to 92% [31].
Ali Balevi et al [40] showed that narrow-band UV-B is an effective and safe alternative for managing extensive and recurrent PV. It is suggested for PV cases, unresponsive to conventional treatments or not suitable for systemic antifungal treatments.
Other reported options include topical tacrolimus 0.03%, showing similar clinical and mycological cure rates to clotrimazole 1% cream in cases of PV [41]. Bartell et al. [42] reported the case of a fourteen-year-old male with recurrent PV, who was treated with isotretinoin for acne vulgaris and had complete remission of the mycosis. This favorable response was probably due to the excessive sebum production in the pathogenesis of PV, allowing for novel therapies.
A prophylactic regimen may delay or avoid PV recurrence. Prophylactic regimens using ketoconazole include 200 mg given on three consecutive days every month or a single dose of 400 mg taken once a month; itraconazole is also a reliable option for prophylaxis [43].
CONCLUSION
The prevention of the recurrence of infections is essential, including superficial infections such as PV. There are currently numerous topical and oral antifungal treatments that effectively reduce the recurrence, leading to clinical and mycological cure. Topical therapy is the first line of treatment for PV in uncomplicated cases of PV. When topical treatment is not feasible or practical, itraconazole and fluconazole are viable options, with pramiconazole as a potential new therapy. In addition to antifungal therapies, non-antifungal treatments have shown efficacy in cases of recurrent PV and could be employed as maintenance or preventive therapy.
The advantages of topical treatment include fast acting, well toleration, smaller risk of severe adverse effects, and limited drug interactions. This is especially evident with the use of ketoconazole. Multiple applications of topical medications may increase adverse events and limit patient compliance, especially in cases of PV in which large body areas are affected. In these cases, oral antifungal may be preferable for many patients, and short courses of oral treatments are the most reliable option. Relapse is common, and thus prophylactic treatment may be necessary to relieve symptoms, especially in recurrent PV.
REFERENCES
1. Harada K, Saito M, Sugita T, Tsuboi R. Malassezia species and their associated skin diseases. J Dermatol. 2015;42:250-7.
2. Framil VM, Melhem MS, Szeszs MW, Zaitz C. New aspects in the clinical course of pityriasis versicolor. An Bras Dermatol. 2011;86:1135-40.
3. Morais PM, Cunha Mda G, Frota MZ. Clinical aspects of patients with pityriasis versicolor seen at a referral center for tropical dermatology in Manaus, Amazonas, Brazil. An Bras Dermatol. 2010;85:797-803.
4. ProhićA, JovovićSadikovićT, Kuskunović-Vlahovljak S, BaljićR. Distribution of malassezia species in patients with different dermatological disorders and healthy individuals. Acta Dermatovenerol Croat. 2016;24:274-81.
5. Saunte DML, Gaitanis G, Hay RJ. Malassezia-associated skin diseases, the use of diagnostics and treatment. Front Cell Infect Microbiol. 2020;10:112.
6. Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev. 2012;25:106-41.
7. Romero-Sandoval K, Costa AA, Teixeira Sousa MG, Furucho CR, Valente N, Criado PR, et al. Recurrent and disseminated pityriasis versicolor:A novel clinical form consequent to Malassezia-host interaction?Med Hypotheses. 2017;109:139-44.
8. Crespo Erchiga V, Ojeda Martos A, Vera Casaño A, Crespo Erchiga A, Sanchez Fajardo F. Malassezia globosa as the causative agent of pityriasis versicolor. Br J Dermatol. 2000;143:799-803.
9. Silva-Rocha WP, de Azevedo MF, Chaves GM. Epidemiology and fungal species distribution of superficial mycoses in Northeast Brazil. J Mycol Med. 2017;27:57-64.
10. Petry V, Tanhausen F, Weiss L, Milan T, Mezzari A, Weber MB. Identification of Malassezia yeast species isolated from patients with pityriasis versicolor. An Bras Dermatol. 2011;86:803-6.
11. Didehdar M, Mehbod AS, Eslamirad Z, Mosayebi M, Hajihossein R, Ghorbanzade B, Khazaei MR. Identification of malassezia species isolated from patients with pityriasis versicolor using PCR-RFLP method in Markazi Province, Central Iran. Iran J Public Health 2014;43:682-6.
12. Park HJ, Lee YW, Choe YB, Ahn KJ. Skin characteristics in patients with pityriasis versicolor using non-invasive method, MPA5. Ann Dermatol. 2012;24:444-52.
13. Cafarchia C, Gasser RB, Figueredo LA, Latrofa MS, Otranto D. Advances in the identification of Malassezia. Mol Cell Probes. 2011;25:1-7.
14. Mayser P, Töws A, Krämer HJ, Weiss R. Further characterization of pigment-producing Malassezia strains. Mycoses. 2004;47:34-9.
15. Rojas FD, Sosa Mde L, Fernández MS, Cattana ME, Córdoba SB, Giusiano GE. Antifungal susceptibility of Malassezia furfur, Malassezia sympodialis, and Malassezia globosa to azole drugs and amphotericin B evaluated using a broth microdilution method. Med Mycol. 2014;52:641-6.
16. Sparber F, LeibundGut-Landmann S. Host responses to Malassezia spp. in the mammalian skin. Front Immunol. 2017;8:1614.
17. Yang YS, Shin MK, Haw CR. Atrophying pityriasis versicolor:Is this a new variant of pityriasis versicolor?Ann Dermatol. 2010;22:456-9.
18. Krämer HJ, Podobinska M, Bartsch A, Battmann A, Thoma W, Bernd A, et al. Malassezin, a novel agonist of the aryl hydrocarbon receptor from the yeast Malassezia furfur, induces apoptosis in primary human melanocytes. Chembiochem. 2005;6:860-5.
19. Nenoff P, Krüger C, Mayser P. Cutaneous malassezia infections and Malassezia-associated dermatoses:An update. Hautarzt. 2015;66:465-84.
20. Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53:137-41.
21. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29.
22. Gupta AK, Lyons DC. Pityriasis versicolor:An update on pharmacological treatment options. Expert Opin Pharmacother. 2014;15:1707-13.
23. Hu SW, Bigby M. Pityriasis versicolor:A systematic review of interventions. Arch Dermatol. 2010;146:1132-40.
24. Gupta AK, Lane D, Paquet M. Systematic review of systemic treatments for tinea versicolor and evidence-based dosing regimen recommendations. J Cutan Med Surg. 2014;18:79-90.
25. Helou J, Obeid G, Moutran R, Maatouk I. Pityriasis versicolor:A case of resistance to treatment. Int J Dermatol. 2014;53:e114-6.
26. *Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev. 2012;25:106-41.
27. Hald M, Arendrup MC, Svejgaard EL, Lindskov R, Foged EK, Saunte DM, et al. Evidence-based Danish guidelines for the treatment of Malassezia-related skin diseases. Acta Derm Venereol. 2015;95:12-9.
28. Leong C, Wang J, Toi MJ, Lam YI, Goh JP, Lee SM, Dawson TL. Effect of zinc pyrithione shampoo treatment on skin commensal Malassezia. Med Mycol. 2021;59:210-213.
29. Rad F, Nik-Khoo B, Yaghmaee R, Gharibi F. Terbinafin 1% Cream and ketoconazole 2% cream in the treatment of pityriasis versicolor:A randomized comparative clinical trial. Pak J Med Sci. 2014;30:1273-6.
30. Muzaffar F, Ilyas M, Suhail M, Ejaz A, Rehman SB, Iqba Z. Keratolytic soaps versus topical azoles in the treatment of pityriasis versicolor. J Pak Assoc Dermatol. 2005;15:313-6.
31. Shi TW, Zhang JA, Tang YB, Yu HX, Li ZG, Yu JB. A randomized controlled trial of combination treatment with ketoconazole 2% cream and adapalene 0.1% gel in pityriasis versicolor. J Dermatolog Treat. 2015;26:143-6.
32. Bakr E, Abdo H, Abd-Elaziz H, Abd-Elrazek H, Amer M. Adapalene gel 0.1% vs ketoconazole cream 2% and their combination in treatment of pityriasis versicolor:A randomized clinical study. Dermatol Ther. 2020;33:e13319.
33. Faergemann J. Pityrosporum infections. J Am Acad Dermatol. 1994;31:S18-20.
34. Leong C, Kit JCW, Lee SM, Lam YI, Goh JPZ, Ianiri G, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65:e01975-20.
35. Berger S, El Chazli Y, Babu AF, Coste AT. Azole resistance in Aspergillus fumigatus:A consequence of antifungal use in agriculture?Front Microbiol. 2017;8:1024.
36. Kano R, Yokoi S, Kariya N, Oshimo K, Kamata H. Multi-azole-resistant strain of Malassezia pachydermatis isolated from a canine Malassezia dermatitis. Med Mycol. 2019;57:346-50.
37. Suzuki C, Hase M, Shimoyama H, Sei Y. Treatment outcomes for Malassezia folliculitis in the dermatology department of a university hospital in Japan. Med Mycol J. 2016;57:E63-6.
38. KarakaşM, Durdu M, Memişoğlu HR. Oral fluconazole in the treatment of tinea versicolor. J Dermatol. 2005;32:19-21.
39. Partap R, Kaur I, Chakrabarti A, Kumar B. Single-dose fluconazole versus itraconazole in pityriasis versicolor. Dermatology. 2004;208:55-9.
40. Balevi A, Üstüner P, Kakşi SA, Özdemir M. Narrow-band UV-B phototherapy:An effective and reliable treatment alternative for extensive and recurrent pityriasis versicolor. J Dermatolog Treat. 2018;29:252-5.
41. Sepaskhah M, Sadat MS, Pakshir K, Bagheri Z. Comparative efficacy of topical application of tacrolimus and clotrimazole in the treatment of pityriasis versicolor:A single blind, randomised clinical trial. Mycoses. 2017;60:338-42.
42. Bartell H, Ransdell BL, Ali A. Tinea versicolor clearance with oral isotretinoin therapy. J Drugs Dermatol. 2006;5:74-5.
43. Faergemann J, Gupta AK, Al Mofadi A, Abanami A, Shareaah AA, Marynissen G. Efficacy of itraconazole in the prophylactic treatment of pityriasis (tinea) versicolor. Arch Dermatol. 2002;138:69-73.
REFERENCES (USED IN TABLE 1)
1. Dyląg M, Leniak E, Gnat S, Szepietowski JC, Kozubowski L. A case of anti- pityriasis versicolor therapy that preserves healthy mycobiome. BMC Dermatol. 2020;20:9.
2. Abdollahimajd F, Niknezhad N, Niknejad N, Nikvar M. Infantile hypopigmented pityriasis versicolor:Two uncommon cases. Turk Pediatri Ars. 2019;54:277-80.
3. Gobbato AA, Babadópulos T, Gobbato CA, Ilha Jde O, Gagliano-JucáT, De Nucci G. A randomized double-blind, non-inferiority Phase II trial, comparing dapaconazole tosylate 2% cream with ketoconazole 2% cream in the treatment of Pityriasis versicolor. Expert Opin Investig Drugs. 2015;24:1399-407.
4. Sharma J, Kaushal J, Aggarwal K. A comparative study of efficacy and safety of eberconazole versus terbinafine in patients of tinea versicolor. Indian J Dermatol. 2018;63:53-56.
5. Ryu HW, Cho JW, Lee KS. Pityriasis versicolor on penile shaft in a renal transplant recipient. Ann Dermatol. 2012;24:345-7.
6. Romano C, Feci L, Mancianti F, Fimiani M. Perineal and genital pityriasis versicolor due to Malassezia globosa. J Eur Acad Dermatol Venereol. 2015;29:1857-8.
7. Marinello E, Piaserico S, Alaibac M. Atrophic pityriasis versicolor occurring in a patient with Sjögren’s syndrome. BMJ Case Rep. 2017;2017:bcr2016218108.
8. DiousséP, Ly F, Bammo M, Lizia S, Diallo TAA, Dione H, et al. Pityriasis versicolor in infants:Unusual clinical presentation and role of corticosteroids used as depigmenting agent for cosmetic purposes in the mother. Pan Afr Med J. 2017;26:31.
9. Sarkar S, Sengupta D, Basak S, Damji SA, Shukla DK, Anurag D. Comparative assessment of the efficacy of topical ketoconazole and topical luliconazole in cases of pityriasis versicolor at a tertiary care hospital in eastern India:A prospective, open, randomized controlled trial. Indian Dermatol Online J. 2016;7:335-6.
10. Day T, Scurry J. Vulvar pityriasis versicolor in an immunocompetent woman. J Low Genit Tract Dis. 2014;18:e71-3.
11. Rad F, Nik-Khoo B, Yaghmaee R, Gharibi F. Terbinafin 1% cream and ketoconazole 2% cream in the treatment of pityriasis versicolor:A randomized comparative clinical trial. Pak J Med Sci. 2014;30:1273-6.
12. Helou J, Obeid G, Moutran R, Maatouk I. Pityriasis versicolor:A case of resistance to treatment. Int J Dermatol. 2014;53:e114-6.
13. Brandi N, Starace M, Alessandrini A, Piraccini BM. Tinea versicolor of the neck as side effect of topical steroids for alopecia areata. J Dermatolog Treat. 2019;30:757-9.
14. Alam HS, Ward JM, Davis LS. Generalized tinea versicolor following initiation of ixekizumab therapy. JAAD Case Rep. 2021;18:54-6.
15. Badri T, Hammami H, Bzioueche N, Zouari B, Mokhtar I. Comparative clinical trial:fluconazole alone or associated with topical ketoconazole in the treatment of pityriasis versicolor. Tunis. 2016;94:107-11.
16. Balestri R, Rech G, Piraccini BM, Antonucci A, Ismaili A, Patrizi A, Bardazzi F. Pityriasis versicolor during anti-TNF-a monoclonal antibody therapy:Therapeutic considerations. Mycoses. 2012;55:444-6.
17. Sharma M, Kansal NK, Gautam RK. A case of Becker’s nevus with pityriasis versicolor. J Eur Acad Dermatol Venereol. 2014;28:1827-8.
18. Jubert E, Martín-Santiago A, Bernardino M, BauzáA. Neonatal pityriasis versicolor. Pediatr Infect Dis J. 2015;34:329-30.
19. Li M, Spaccarelli N, Kendra K, Wu RC, Verschraegen C. Refractory dermatitis contributed by pityriasis versicolor:A case report. J Med Case Rep. 2021;15:212.
20. El-Housiny S, Shams Eldeen MA, El-Attar YA, Salem HA, Attia D, Bendas ER, et al. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor:Formulation and clinical study. Drug Deliv. 2018;25:78-90.
21. Romero-Sandoval K, Costa AA, Teixeira Sousa MG, Furucho CR, Valente N, Criado PR, et al. Recurrent and disseminated pityriasis versicolor:A novel clinical form consequent to Malassezia-host interaction?Med Hypotheses. 2017;109:139-44.
22. Choi E, Tan CL, Aw D. Malassezia:A case of coexisting pityriasis versicolor and Malassezia folliculitis. Singapore Med J. 2020;61:221.
23. Allegue F, Fachal C, González-Vilas D, Zulaica A. Atrophying pityriasis versicolor. Actas Dermosifiliogr (Engl Ed). 2018;109:455-7.
24. Cam VT, Van TN, Hau KT, Huu DL, Minh PPT, Huu SN, et al. Efficacy of azole antifungal in treatment of pityriasis versicolor. Open Access Maced J Med Sci. 2019;7:272-4.
25. Wahab MA, Kamal SB, Shahin MR, Siddique RU, Hassan MR, Hassan BS, et al. Efficacy of itraconazole in the prevention of recurrence of tinea versicolor:A three year follow up. Mymensingh Med J. 2020;29:351-6.
26. Bossini B, Mazzolai M, Berti I, Barbi E. Hyperpigmented pityriasis versicolor misdiagnosed as acanthosis nigricans. Arch Dis Child. 2022;107:92.
27. Cantrell WC, Elewksi BE. Can pityriasis versicolor be treated with 2% ketoconazole foam?J Drugs Dermatol. 2014;13:855-9.
28. Dehghan M, Akbari N, Alborzi N, Sadani S, Keshtkar AA. Single-dose oral fluconazole versus topical clotrimazole in patients with pityriasis versicolor:A double-blind randomized controlled trial. J Dermatol. 2010;37:699-702.
29. Carmo ES, Pereira Fde O, Cavalcante NM, Gayoso CW, Lima Ede O. Treatment of pityriasis versicolor with topical application of essential oil of Cymbopogon citratus (DC) Stapf:Therapeutic pilot study. An Bras Dermatol. 2013;88:381-5.
30. Bakr E, Abdo H, Abd-Elaziz H, Abd-Elrazek H, Amer M. Adapalene gel 0.1% vs ketoconazole cream 2% and their combination in treatment of pityriasis versicolor:A randomized clinical study. Dermatol Ther. 2020;33:e13319.
31. Balevi A, Üstüner P, Kakşi SA, Özdemir M. Narrow-band UV-B phototherapy:An effective and reliable treatment alternative for extensive and recurrent pityriasis versicolor. J Dermatolog Treat. 2018;29:252-5.
32. Shi TW, Zhang JA, Tang YB, Yu HX, Li ZG, Yu JB. A randomized controlled trial of combination treatment with ketoconazole 2% cream and adapalene 0.1% gel in pityriasis versicolor. J Dermatolog Treat. 2015;26:143-6.
33. Khattab FM, Omran FH. 308-nm excimer laser:A hopeful and optional therapy for pityriasis versicolor. J Dermatolog Treat. 2021;32:795-9.
34. Nashwa RK, Ahmed EB, Nemr WA. Comparative study between topically applied irradiated human amniotic membrane in combination with tea tree oil versus topical tioconazole in pityraisis versicolor treatment. Cell Tissue Bank. 2020;21:313-20.
35. Sepaskhah M, Sadat MS, Pakshir K, Bagheri Z. Comparative efficacy of topical application of tacrolimus and clotrimazole in the treatment of pityriasis versicolor:A single blind, randomised clinical trial. Mycoses. 2017;60:338-42.
36. Alberdi E, Gómez C. Successful treatment of pityriasis versicolor by photodynamic therapy mediated by methylene blue. Photodermatol Photoimmunol Photomed. 2020;36:308-12.
37. Lone AH, Ahmad T, Anwar M, Sofi G. Clinical efficacy and safety of a pharmacopial polyherbal Unani formulation in pityriasis versicolor:A comparative randomized single-blind study. J Altern Complement Med. 2012;18:978-82.
38. Jowkar F, Jamshidzadeh A, Pakniyat S, Namazi MR. Efficacy of nitric oxide-liberating cream on pityriasis versicolor. J Dermatolog Treat. 2010;21:93-6.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0001-9306-1619 http://orcid.org/0000-0001-9306-1619 http://orcid.org/0000-0003-2045-3317 http://orcid.org/0000-0003-2045-3317 |




Comments are closed.