Clinical and onychoscopic evaluation of nail changes in psoriasis at a tertiary-care hospital: A cross-sectional study
Rajesh Khokhar1, Rajesh Dutt Mehta1, Bhikam Chand Ghiya1, Prasoon Soni1, Chitralekha Dhaka2, Manoj Kumar Yadav1, Vishnu Jangir1, Aakanksha Arora1, Sumiti Pareek1, Alpana Mohta 1
1
1Department of Dermatology, Venereology and Leprology, Sardar Patel Medical College, Bikaner, Rajasthan, India, 2Department of Pathology, Sardar Patel Medical College, Bikaner, Rajasthan, India
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Up to 30–50% of cases of psoriasis experience nail involvement, while 5–10% may have only isolated nail disease. Recently, the use of onychoscopy, a non-invasive technology, has emerged as a promising tool that may eliminate the necessity of a nail biopsy in most cases.
Objective: The objective of this study was to assess the onychoscopic characteristics of the nail unit in individuals with nail psoriasis.
Materials and Methods: The study recruited fifty patients with a clinical diagnosis of nail psoriasis. Each nail underwent onychoscopic assessment. The clinical degree of cutaneous and nail involvement was evaluated with the Psoriasis Area and Severity Index (PASI) and the Nail Psoriasis Severity Index (NAPSI), respectively.
Results: The following findings were observed significantly more often on onychoscopy than with the naked eye: pitting (p = 0.03), leukonychia (p = 0.04), onycholysis (p = 0.04), and splinted hemorrhage (p = 0.05). The other novel findings included fuzzy lunula, which was only onychoscopically (8%), We also encountered 4% of cases of triangular onychomadesis and 6% of non-traumatic Median canaliform dystrophy of Heller.
Conclusion: Our findings suggest that, even before clinical symptoms become obvious, onychoscopy may help with nail lesion diagnosis.
Key words: Onychoscopy; Nail Psoriasis; Psoriasis; Nail Pitting; Fuzzy Lunula; Triangular Onychomadesis
INTRODUCTION
Psoriasis is a chronic, papulosquamous disorder with an immune-mediated pathogenesis. The disease may involve not only the skin, yet also the nails and mucosa. The prevalence of psoriasis varies over the world, affecting between 0.5% and 2.5% of the global population [1,2]. In around 30–50% of cases, nail affliction is observed, with a lifetime prevalence between 80% and 90%. Nail involvement is widely prevalent in psoriatic arthropathy (PsA) and severe plaque psoriasis [3,4]. In fact, 5–10% of psoriatic individuals typically have isolated nail disease [5,6].
It is fairly simple to detect nail psoriasis when skin lesions are present. Although, due to its resemblance to other causes of dystrophic nails, including onychomycosis, lichen planus pityriasis rubra pilaris, alopecia areata, and traumatic onycholysis, there is a diagnostic conundrum in cases with solitary nail involvement [7]. Despite the high specificity of nail biopsies in diagnosing nail diseases, they have a low sensitivity and may proficiently diagnose nail psoriasis in only 50% of cases [3,8].
Dermoscopy is a quick, non-invasive, and in vivo technique with proven diagnostic value for pigmented skin and nail abnormalities. Dermoscopy is also one of the most effective methods for spotting early nail involvement. This property may eliminate the need for histopathological analysis in nail psoriasis diagnosis and treatment follow-up.
To date, only a handful of authors have attempted to examine the dermoscopic characteristics of nail psoriasis [9–12]. In this study, we intended to assess the value of onychoscopy in nail psoriasis. Our primary objective was to observe the onychoscopic findings in the nails of the psoriatic patient. The secondary objectives included assessing the prognostic significance of subclinical nail changes visualized on the onychoscope and correlating the severity of the disease with onychoscopic findings.
MATERIALS AND METHODS
This prospective and observational study was conducted at outpatient and inpatient dermatology departments over the course of one year after obtaining due approval from the institutional ethics committee (F.29.(Acad)SPMC/2021/3014). All participants gave written informed consent to be a part of the study. The study population included patients with a clinical diagnosis of psoriasis. In clinically ambiguous cases, histopathological analysis was performed to confirm the diagnosis. Only those cases who had psoriasis with nail lesions or isolated nail psoriasis were enrolled. Patients with a significant systemic illness or another concurrent dermatological or cutaneous disease were excluded. A thorough medical history was taken, and general physical examination and systemic and joint examinations were performed.
In addition to clinical and onychoscopic examinations, photographic documentation was completed on all 20 nails (10 fingers and 10 toenails) with a handheld dermatoscope (DermLite, 3rd generation; magnification: 10×) under the supervision of a senior faculty. Digital photographs were taken with a mobile camera with the same settings. The images were examined on the computer screen and, after thorough image analysis, onychoscopic findings were interpreted and noted.
Calculating the mean and standard deviations for the continuous variables served as descriptive statistics. Categorical variables were displayed as percentages and absolute numbers. In order to compare nominal categorical data between the groups, the chi-squared goodness-of-fit test was employed. The Pearson correlation coefficient was employed to calculate the correlation between the different variables.
Considering the prevalence of psoriasis in India at 2.8%, a clinical involvement of the nails in 60% of cases, a confidence level of 50%, and a 5% margin of error, a sample size of 31 was calculated [13,14]. However, we increased the sample size to 50 in view of potential subclinical nail involvement visualizable on the onychoscope.
Data was entered, checked, and analyzed with SPSS 22.0. The chi-squared test and Z scores were calculated wherever necessary. A p value < 0.05 was considered statistically significant.
The clinical degree of cutaneous and nail involvement was evaluated with the PASI and NAPSI, respectively.
RESULTS
In our study, a total of fifty patients with psoriasis were included, ranging from 14 years to 71 years. The majority of the psoriatic patients were between 20–29 years of age (26%), with a male-to-female ratio of 1.78:1. The mean age of our study subjects was 39.66 ± 19.06 years. The demographic data of the patients was tabulated in Table 1. In this study, 64% of the patients were diagnosed with chronic plaque psoriasis (CPP), followed by 14% of cases with palmoplantar psoriasis (PPP), 10% of cases with erythrodermic psoriasis (EP), 6% of cases with nail psoriasis (NP), 4% of cases with guttate psoriasis (GP), and 2% of cases with inverse psoriasis (IP).
Clinically, a majority of the cases had the involvement of ≤ 5 nails (38%), followed by the involvement of 6–10 nails (30%), and of 11–15 nails (26%) The average number of nails involved per case was 7.8 nails. Similarly, onychoscopically, a majority of the cases had the involvement of ≤ 5 nails (38%), followed by the involvement of 6–10 nails (30%), and of 11–15 nails (20%). The onychoscope had a higher proficiency in detecting a higher number of nails, yet the difference was statistically insignificant (0.71) (Table 2).
A majority of the patients (n = 28; 56%) had NAPSI scores ranging from 0 to 20, followed by 14% with scores of 21–40, 10% with scores of 41–60, 10% with scores of 61–80, 6% with scores of 81–100, and 4% with scores of 101–120.
In our study, the following nail matrix findings were seen: 68% had pitted nails, 44% had leukonychia, 48% had crumbling, 8% had onychomadesis, 8% had fuzzy lunula, 6% had median canaliform dystrophy, and 6% had trachyonychia. However, onychoscopically, a greater number of patients had nail matrix findings. Significant p-value results were found with the chi-squared test for pitting (p = 0.03) and leukonychia (p = 0.04), with onychoscopy being superior to clinical examination for the identification of these nail matrix findings (Table 3).
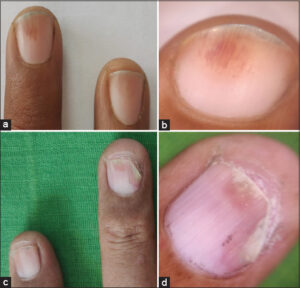
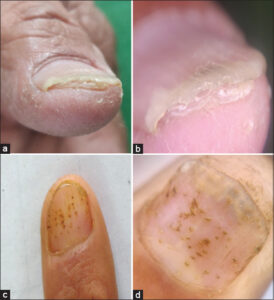
The following nail bed findings were seen clinically: 54% had onycholysis, 42% had SUHK, 20% had splinter hemorrhage (Figs. 1a and 1b), and 14% had oil drop signs (Figs. 1c, 1d, 2a, and 2b). On onychoscopy, significant p-value results were found with the chi-squared test for onycholysis (p = 0.04) and splinter hemorrhage (p = 0.05), with onychoscopy being superior to clinical examination for the identification of these nail bed findings (Table 4).
Overall, the mean NAPSI of the patients was 31.46. There was a strong positive correlation between NAPSI and duration of psoriasis. The correlation coefficient was 0.82, and the p value was < 0.0001 (highly significant correlation). There was also a moderate correlation between NAPSI and PASI. The correlation coefficient was 0.67, and the p value was < 0.0001 (highly significant correlation).
DISCUSSION
In our study, the mean age of the study subjects was 39.66 ± 19.06 years (ranging from 18 to 79 years), which was comparable to similar studies conducted across the globe. A study by Yadav et al. reported a mean age of 38.36 years [10]. Similar results were reported by Wanniang et al. (45.02 years) and Daulatabad et al. (36.3 years) [9,14], together with studies by van der Velden et al. (48 years), Marina et al. (51.89 years), Brazzelli et al. (52.53 years), and Hashimoto et al. (47.5 years) [15–18]. There was also a male preponderance in this study, with a male-to-female ratio of 1.78:1. Fairly similar results were reported by Chauhan et al., Hashimoto et al., and Yadav et al. [3,10,18].
In our study, the most common nail finding was pitting (Figs. 2c and 2d), followed by onycholysis and crumbling. These results were consistent with previously conducted research [3,9,14]. However, red lunula, a relatively uncommon nail finding in psoriatic patients, was not observed in any of our cases. Our subjects’ mean PASI score was 8.93 ± 5.04 (0–33), which was comparable to that observed by Chauhan et al. However, Wanniang et al., Brazzelli et al., Schons et al., and Daulatabad et al. observed a higher mean PASI [9,14,17,19]. The mean NAPSI was 31.46, which was comparable to similar studies [14,19,20]. NAPSI was also comparable in individuals with associated arthritis, and scores were greater in the fingernails than the toenails.
In this study, we also found a positive correlation between NAPSI and PASI scores, as well as between NAPSI and disease duration. This data was a strong indicator that nail involvement becomes more pronounced with increasing disease duration and severity. Although Rich et al. reported no significant correlation between NAPSI and PASI, both Chuhan et al. and Patsatsi et al. detected a significant correlation [3,21,22]. This was attributed to the fact that, while Chauhan et al. assessed the total NAPSI (0–160) the way that we did, Rich et al. used only the target NAPSI (0–8) [3,21]. As far as the correlation between disease duration and NAPSI was concerned, Daulatabad et al. found a significant correlation between the two variables. Wanniang et al. also reported a significantly positive correlation between dermatoscopic NAPSI and PASI scores [9,14].
On onychoscopic evaluation, pitting was the most common nail matrix finding, observed in 43 (86%) cases. There was a significantly higher prevalence of pitting on onychoscopy than clinically (p = 0.03). This finding was in agreement with observations made by Chauhan et al. Yadav et al., Yorulmaz et al., and Wanniang et al. [3,9,10,12].
Other findings, such as leukonychia (p = 0.04), onycholysis (p = 0.05), and splinter hemorrhage (p = 0.04), were also significantly higher on onychoscopy. Other onychoscopic findings of the nail matrix and nail bed findings were comparable to the naked eye examination.
Leukonychia and nail plate crumbling were the two most common nail findings, observed at a significantly higher rate on onychoscopy than clinically. These findings were also reported by Wanniang et al. and Yorulmaz et al [9,12].
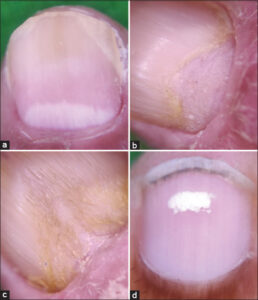
A novel finding of this study was fuzzy lunula (Fig. 3a), which was observed only onychoscopically. Fuzzy lunula was seen in 4 (8%) cases. Fairly unprecedented in the literature, fuzzy lunula was a unique finding in this study, which had previously been reported only once, by Chauhan et al. [3]. Onychoscopically, the nail lesion appeared as a wide and crooked, white lunula.
We also encountered two cases of triangular onychomadesis and three of non-traumatic median canaliform dystrophy of Heller (Figs. 3b – 3d). To date, these findings have not been reported by any other study.
Our study was limited by a small sample size, a short study duration, and the absence of histopathological or radiological correlation. Additionally, the impact of the treatment on nail findings was not assessed.
CONCLUSION
In conclusion, the nails are indeed a window to the underling disorders of the body [23,24]. Dermoscopy serves as a bridge between histological and clinical examinations and aids in the identification of nail psoriasis well before the clinical indications of nail involvement are obvious. A possible early indicator of disease activity is the appearance of dermoscopic characteristics in apparently healthy nails. In patients with nail psoriasis, this study thoroughly detailed the dermoscopic characteristics of the nail matrix and nail bed. However, further controlled investigations need to be conducted on a larger sample size in order to proficiently determine the sensitivity and specificity of all dermoscopic features in order to standardize their diagnostic value.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Dogra S, Yadav S. Psoriasis in India:Prevalence and pattern. Indian J Dermatol Venereol Leprol. 2010;76:595-601.
2. Gudjonsson JE, Karason A, Runarsdottir EH, Antonsdottir AA, Hauksson VB, Jónsson HH, et al. Distinct clinical differences between HLA-Cw*0602 positive and negative psoriasis patients:An analysis of 1019 HLA-C- and HLA-B-typed patients. J Invest Dermatol. 2006;126:740-5.
3. Chauhan A, Singal A, Grover C, Sharma S. Dermoscopic features of nail psoriasis:An observational, analytical study. Skin Appendage Disord. 2020;6:207-15.
4. Singh S, Sood A, Sinha P, Joshi R, Patrikar S, Das P. Evaluating the extent of agreement between the EARP (Early Arthritis for Psoriatic Patients) and PEST (Psoriasis Epidemiology Screening Tool) questionnaires in screening for psoriatic arthropathy in patients with psoriasis in a tertiary-care dermatology outpatient department. Our Dermatol Online. 2020;11:346-50.
5. De Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-62.
6. Jiaravuthisan MM, Sasseville D, Vender RB, Murphy F, Muhn CY. Psoriasis of the nail:Anatomy, pathology, clinical presentation, and a review of the literature on therapy. J Am Acad Dermatol. 2007;57:1-27.
7. Dogra A, Arora AK. Nail psoriasis:The journey so far. Indian J Dermatol. 2014;59:319-33.
8. Puri N, Kaur T. A study of nail changes in various dermatosis in Punjab, India. Our Dermatol Online. 2012;3:164-70.
9. Wanniang N, Navya A, Pai V, Ghodge R. Comparative study of clinical and dermoscopic features in nail psoriasis. Indian Dermatol Online J. 2020;11:35-40.
10. Yadav TA, Khopkar US. Dermoscopy to detect signs of subclinical nail involvement in chronic plaque psoriasis:A study of 68 patients. Indian J Dermatol. 2015;60:272-5.
11. Iorizzo M, Dahdah M, Vincenzi C, Tosti A. Videodermoscopy of the hyponychium in nail bed psoriasis. J Am Acad Dermatol. 2008;58:714-5.
12. Yorulmaz A, Artuz F. A study of dermoscopic features of nail psoriasis. Postepy Dermatol Alergol. 2017;34:28-35.
13. Thappa DM, Munisamy M. Research on psoriasis in India:Where do we stand?Indian J Med Res. 2017;146:147-9.
14. Daulatabad D, Grover C, Kashyap B, Dhawan AK, Singal A, Kaur IR. Clinical and serological characteristics of nail psoriasis in Indian patients:A cross-sectional study. Indian J Dermatol Venereol Leprol. 2017;83:650-5.
15. van der Velden HM, Klaassen KM, van de Kerkhof PC, Pasch MC. Fingernail psoriasis reconsidered:A case-control study. J Am Acad Dermatol. 2013;69:245-52.
16. Marina EM, Botar-Jid C, Bolboaca SD, Roman, Senila CS, Mihu CM, et al. Patterns of clinical nail appearances in patients with cutaneous psoriasis. Clujul Med. 2017;90:22-7.
17. Brazzelli V, Carugno A, Alborghetti A, Grasso V, Cananzi R, Fornara L, et al. Prevalence, severity and clinical features of psoriasis in fingernails and toenails in adult patients:Italian experience. J Eur Acad Dermatol Venereol. 2012;26:1354-9.
18. Hashimoto Y, Uyama M, Takada Y, Yoshida K, Ishiko A. Dermoscopic features of nail psoriasis treated with biologics. J Dermatol. 2017;44:538-41.
19. Schons KR, Beber AA, Beck MO, Monticielo OA. Nail involvement in adult patients with plaque-type psoriasis:Prevalence and clinical features. An Bras Dermatol. 2015;90:314-9.
20. Sharada RG, Thomas J. A study on psoriasis of nails-severity scoring system. ARC J Dermatol. 2016;1:13-6.
21. Rich P, Griffiths CE, Reich K, Nestle FO, Scher RK, Li S, et al. Baseline nail disease in patients with moderate to severe psoriasis and response to treatment with infliximab during 1 year. J Am Acad Dermatol. 2008;58:224-31.
22. Patsatsi A, Kyriakou A, Sotiriadis D. Ustekinumab in nail psoriasis:An open-label, uncontrolled, nonrandomized study. J Dermatolog Treat. 2013;24:96-100.
23. Yorulmaz A, Yalcin B. A novel dermoscopic feature in traumatic onycholysis. Our Dermatol Online. 2018;9:307-9.
24. Molina-Hernandez AL, Ramírez-Marín HA, Bonifaz A, Dominguez-Cherit JG. Onychomycosis in patients with diabetes mellitus:Etiology, clinical features, and treatment response. Our Dermatol Online. 2021;12:359-66.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0001-7526-2089 http://orcid.org/0000-0001-7526-2089 |





Comments are closed.