DRESS syndrome: A descriptive series of 62 cases
Zoubida Mehsas , Ibtissam Boubnane, Soukaina Sektaoui, Meriame Meziane, Nadia Ismaili, Leila Benzekri, Karima Senouci
, Ibtissam Boubnane, Soukaina Sektaoui, Meriame Meziane, Nadia Ismaili, Leila Benzekri, Karima Senouci
Department of Dermatology and Venereology, CHU Ibn Sina, Mohamed V University of Rabat, Morocco
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Drug reactions with eosinophilia and systemic symptoms (DRESS) are rare, yet potentially life-threatening, adverse drug reactions. This retrospective study aimed to analyze the epidemiological, etiological, therapeutic, and evolutionary characteristics of DRESS in the context of the dermatology department of Ibn Sina Hospital in Rabat, Morocco.
Methods: A retrospective descriptive study was conducted over a period of fourteen years (January 2009 thru December 2022). All archived records of patients hospitalized for DRESS syndrome at the dermatology department of Ibn Sina University Hospital were collected. Cases were identified with the RegiSCAR criteria and, for each patient, epidemiological, anamnestic, clinical, paraclinical, therapeutic, and evolutionary data was collected.
Results: The study included 62 patients, with a female predominance (67.7%). The average age was 48.59 years. The average time to onset of symptoms after drug intake was 27 days, and the duration of symptoms after the discontinuation of the suspected drug was more than two weeks in 80% of the cases. Clinically, all patients had pruritus and maculopapular rash, and 78% had erythroderma. Facial swelling was found in 66.1% of the cases, and 56.4% presented with at least one mucosal involvement. Hematological abnormalities consisted of hypereosinophilia in 85.36%. The most commonly implicated drugs were allopurinol, phenobarbital, and Salazopyrine.
Conclusion: This study provides important epidemiological, etiological, clinical, therapeutic, and evolutionary data of patients hospitalized for DRESS syndrome in the context of the dermatology department of Ibn Sina Hospital in Rabat. The results of the study may be used to improve the diagnosis and management of DRESS syndrome in similar settings.
Key words: Toxidermia; Dress Syndrome; Epidemiology
INTRODUCTION
Toxidermias are undesirable drug effects that may be potentially serious [1]. Severe forms include acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens–Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN), also known as Lyell’s syndrome [2].
DRESS syndrome is a rare drug-induced idiosyncratic hypersensitivity reaction that combines skin manifestations and systemic involvement [3].
In this retrospective study conducted at the dermatology department of Ibn Sina Hospital in Rabat, we aimed to analyze the epidemiological, etiological, therapeutic, and evolutionary characteristics of DRESS syndrome in our context by comparing our data to the literature.
MATERIALS AND METHODS
This was a retrospective, descriptive study covering a period of fourteen years (January 2009 thru December 2022). All archived records of patients hospitalized for DRESS syndrome at the dermatology department of Ibn Sina University Hospital were collected. Cases were identified with the RegiSCAR criteria, which allows cases to be classified as possible, probable, certain, and excluded. All cases classified as possible, probable, or certain were included. For each patient, we collected epidemiological, anamnestic, clinical, paraclinical, therapeutic, and evolutionary data.
Ethics Statement
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Informed consent for participation in this study was obtained from all patients.
RESULTS
We collected 62 patients, 42 females (67.7%) and 20 males (32.3%), indicating a clear female predominance with a male-to-female sex ratio of 0.47. The average age was 48.59, ranging from 22 to 75 years.
Fifty-four patients (87.80%) had at least one medical history, with the most frequent ones being: arterial hypertension found in 15 patients (27.7%), autoimmune diseases (rheumatoid arthritis, ankylosing spondylitis, ulcerative colitis) found in 8 (14.8%), diabetes in 6 (9.67%), heart disease in 7 (12.96%), recent neurosurgical intervention (extra-dural hematoma, spontaneous cerebral hematoma, schwannoma, pituitary adenoma) in 4 (6.45%), depression in 4 (6.45%), chronic renal failure in 4 (6.45%), head trauma in 4 (6.45%), and epilepsy in 2.
The average time to the onset of symptoms after drug intake was 27 days. The duration of symptoms after the discontinuation of the suspected drug was more than two weeks in 80% of the cases.
Clinically, pruritus was noted in all patients. Fever was observed in 68.29%, and fatigue in 32 (51.61%). Burning sensations were reported in four patients, dysphagia was in three, dyspnea in two, and dysphonia in one.
A maculopapular rash was present in all patients, among which 78% had erythroderma. Facial swelling was found in 41 patients (66.1%), scaling in 23 (37.09%), purpura in 21 (33.8%), pustules in 7 (11.29%), skin erosions in 5, and vesicular lesions in 2. Thirty-five patients (56.4%) presented with at least one mucosal involvement. The oral mucosa was the most affected, mainly with cheilitis, in 17 cases (48.57%). Stomatitis was noted in 6 cases. Conjunctivitis was noted in 9 cases (25.71%), oral erosions in 2 cases, and genital erosions in 2 cases (Graph 1).
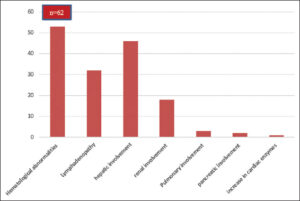
Lymphadenopathy was present in 51.22% of the cases. Hematological abnormalities consisted of hypereosinophilia in 85.36% of the cases, leukocytosis in 63.2%, and abnormal lymphocytes in 26.7%. Visceral involvement was dominated by hepatic involvement, present in 75% of the cases, followed by renal involvement in 29.26%. Pulmonary involvement was noted in 3 cases, pancreatic involvement in 2, and an increase in cardiac enzymes in one (Graph 2).
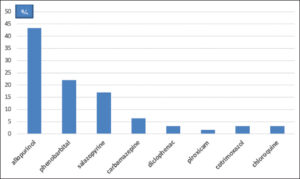
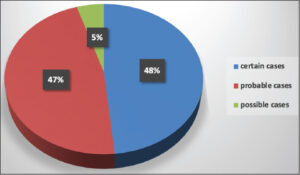
RegiSCAR score calculation identified 30 certain cases, 29 probable cases, and 3 possible cases (Graph 3). The most commonly implicated drugs were allopurinol (43.34%), phenobarbital (22%), and Salazopyrine (17%). All implicated drugs were prescribed by a medical professional. Allopurinol was prescribed by various physicians (general practitioners, cardiologists, nephrologists, rheumatologists, traumatologists, and endocrinologists) for hyperuricemia in 18 cases, suspected gout in 5, and actual gout attack in 4. Phenobarbital was prescribed by neurosurgeons prophylactically after a neurosurgical procedure in 8 cases and after a head trauma in 4. It was only indicated for epilepsy treatment in 2 cases. Salazopyrine was indicated for the treatment of chronic inflammatory rheumatism and ulcerative colitis. Carbamazepine was prescribed by psychiatrists for neurogenic pain and psychiatric disorders (Graph 4).
The discontinuation of the suspected medication(s) as well as any non-essential medication was recommended for all patients. Local care and emollients were also recommended for all our patients.
Hydroelectrolytic resuscitation was indicated in 12 patients (29%), mainly in cases of renal impairment or dehydration.
Treatment was mainly based on oral corticosteroids, which were indicated in 63.4% of the cases. The corticosteroid doses ranged from 0.5 to 1 mg/kg/day depending on the severity of systemic involvement. The total duration of corticosteroid therapy was estimated to be around one year.
The evolution was favorable in 95.1% of the cases. However, three deaths were recorded (Table 1).
Short-term complications were mainly infectious: one case of pneumonia, two cases of nosocomial urinary tract infection, and three cases of soft tissue infection (abscess, lymphangitis, and erysipelas), which responded well to antibiotic therapy, yet with worsening rash and cytolysis in two cases.
Long-term complications could not be determined due to the retrospective nature of our study. One case of thyroiditis was noted (patient readmitted to our department). One case of relapse was reported four months after the discontinuation of corticosteroid therapy.
DISCUSSION
Drug rash with eosinophilia and systemic symptoms (DRESS syndrome), also known as drug-induced hypersensitivity syndrome, is an uncommon severe systemic hypersensitivity drug reaction. It is estimated to occur in 1 for every 1000 to 10,000 drug exposures [1]. It may affect patients of all ages and typically presents 2 to 6 weeks after exposure to the culprit medication [4].
Several reports state that, generally, there is no sex predominance. Mizukawa et al. described a female predominance with a male-to-female ratio of 0.71 [4]. In our study, we also noted a clear female predominance, with a male-to-female ratio of 0.46.
There is also no seasonal variability or specific atopic background in patients. However, Mizukawa et al. reported a history of mainly viral infection in half of their patients in the month preceding DRESS syndrome [5].
Its complex pathophysiology is now better understood, involving a predisposing immunogenetic background and a dominant reactivation of herpesviruses, specifically the HHV6 virus. Its pathophysiology has been clarified by the demonstration of reactivations of herpes viruses, which explains the seriousness of the clinical manifestations, in particular, the systemic attacks, as well as the biological modifications of DRESS syndrome [6,7].
DRESS syndrome has some highly specific characteristics, particularly chronological. Patients typically develop symptoms three weeks after the initiation of the triggering drug. The latency period of DRESS syndrome is longer than that of other delayed hypersensitivity drug reactions (SJS/NET, PEAG, fixed pigmentary erythema, maculopapular rash). The duration of symptoms is also prolonged, generally lasting more than fifteen days after the discontinuation of the implicated drug, with an episodic course that may be interspersed with periods of remission [8].
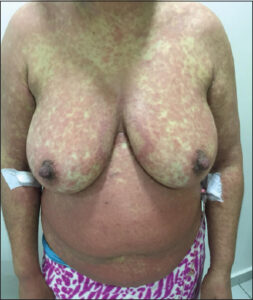
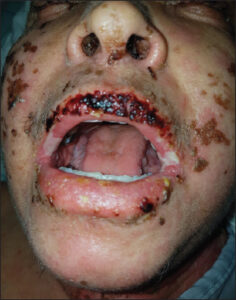
The clinical presentation is highly polymorphic, characterized by the presence of fever, ranging from 38°C to over 40°C, which evolves in spikes. The fever is present in 90% to 100% of cases. A more pronounced fever is found in cases of erythroderma. Fever usually precedes the skin rash by several days, yet both symptoms may occur simultaneously [9,10]. Pruritus is also almost always present. Dysphagia may also be associated. The general condition is usually impaired. Some authors describe a high frequency of prodromes, such as upper respiratory tract infections, which they believe could support the role of viral infections [11]. In our series, pruritus was present in all patients. Fever was observed during hospitalization in 42 patients (68%), and 5 other patients reported febrile sensations before hospitalization. Dysphagia was reported in 3 cases. Skin rash is the most frequent clinical sign, found in 73–100% of cases. It usually begins on the face, trunk, and root of the limbs. The cutaneous signs are variable, often consisting of a morbilliform exanthema that is difficult to distinguish from a benign toxidermia with a maculopapular rash. The eruption generally affects more than 50% of the skin surface (Fig. 1). The maculopapular elements may merge to form infiltrated erythematous plaques sometimes with a purpuric evolution. The skin rash usually becomes generalized and evolves toward a severe exfoliative phase with significant desquamation, especially on the legs and feet. Other clinical aspects may also be observed, most often with a polymorphic eruption with atypical target lesions, vesiculobullous lesions, erosions, or lichenoid, pustular, urticarial, or eczematous elements. Desquamation marks the phase of symptom resolution (Fig. 2) [12]. In our series, a maculopapular rash was present in all our patients, with 78% having erythroderma. Purpura was found in 33.87% of the cases, and pustules were present in 7 patients. Facial edema, predominant in the periorbital region, is characteristic of DRESS syndrome. It is seen in 50–76% of cases [9]. It was present in 75% of our patients. Mucosal involvement is described as cheilitis, pharyngeal erythema, or even tonsillar hypertrophy manifested by odynophagia. Conjunctivitis is possible as well as oral or genital aphthous lesions. These conditions may be seen in more than 50% of cases. Infiltration of the salivary glands leading to xerostomia may also be observed [13]. In our series, cheilitis was the most frequently observed sign (41.46%) (Fig. 3), followed by conjunctivitis (21.95%). Oral erosions were noted in two cases and genital erosions in two.
Hematological abnormalities are mainly characterized by hypereosinophilia, leukocytosis, hypereosinophilia, as well as the presence of hyperbasophilic lymphocytes. Hypereosinophilia is usually associated with organ involvement. In our series, leukocytosis was noted in 63% of our patients, hypereosinophilia was greater than 1500 in 75% of patients and between 900 and 1500 in 4 cases. The presence of atypical lymphocytes was noted in 27% of our cases. Other systemic involvements may also occur (lymph nodes, liver, lung, kidney, heart, neurological, digestive, endocrine), which contribute to the severity of the syndrome.
The histopathological features of DRESS syndrome are generally non-specific. In recent years, several commonly encountered histopathological patterns of DRESS syndrome have been identified, including spongiosis, interface dermatitis, vascular abnormalities, and superficial and perivascular infiltrate [14].
More than forty drugs have been reported to be associated with DRESS syndrome. In 2013, a prospective study by RegiSCAR on 117 patients reported that anti-epileptics, particularly carbamazepine, were the most commonly implicated drugs (37%), followed by allopurinol (18%). Other drugs traditionally associated with DRESS syndrome were much less common (Sulfasalazine in 8 cases, vancomycin in 7, minocycline in 6, dapsone in 3)[15]. In our study, the most commonly implicated drug was allopurinol (46.34%), followed by phenobarbital (22%), and sulfasalazine (17%). Carbamazepine was only implicated in four cases.
Regarding prognosis, DRESS syndrome is a potentially fatal severe drug reaction with a mortality rate of approx. 10% [4]. In our series, three deaths were noted, resulting in a rate of 7.3%. The course of DRESS syndrome is variable and unpredictable. A higher risk of severe systemic involvement was reported in DRESS syndrome induced by allopurinol and minocycline than by other medications [12]. However, organ damage may be highly severe in some patients, resulting in the permanent impairment of the function of the affected organs [13]. Liver transplants have been recommended for patients with severe liver injury. Patients with underlying chronic renal disease are subject to marked and permanent deterioration of renal function, sometimes requiring lifelong hemodialysis [16].
Thyroid disorders are the most commonly reported long-term sequela of DRESS syndrome, with a rate of 4.8%. These mainly include Graves’ disease, Hashimoto’s thyroiditis, and subacute lymphocytic thyroiditis. The reactivation of HHV-6 is also believed to play an important role in the development of these thyroiditis conditions [17].
In 2018, the reference center for toxic bullous dermatoses and severe drug-induced skin reactions (FISARD: French Investigators for Skin Adverse Reactions to Drugs) proposed a therapeutic scheme for the management of severe drug-induced skin reactions, including DRESS syndrome. The discontinuation of the implicated drug, hospitalization during the acute phase, and clinical and paraclinical evaluations are always necessary [18].
Following the initial assessment, patients may be classified according to the severity of the various systemic manifestations of DRESS syndrome into mild, moderate, or severe cases. Severe DRESS syndrome justifies urgent general corticosteroid therapy, the modalities of which (methylprednisolone bolus 500 mg/day for three days followed by oral prednisone 1 mg/kg, or prednisone 1 mg/kg from the outset) are not consensus-based. In the case of moderate-severity DRESS syndrome, a trial comparing the efficacy of local corticosteroid therapy with clobetasol propionate and general corticosteroid therapy at a dose of 0.5 mg/kg/day of prednisone is underway in France. Minor DRESS syndrome may be treated with local corticosteroid therapy, such as clobetasol propionate at a dose of 30 g/day until the dermatological and systemic manifestations are controlled, followed by gradual tapering over 3 to 6 months [19].
Regardless of its modality (local or general), corticosteroid therapy should be prolonged (3 to 6 months) with slow tapering to prevent relapses. Other therapeutic modalities, such as antivirals and IVIG, are no longer recommended [20].
CONCLUSION
DRESS syndrome is a serious toxidermia that may be life-threatening and functionally dangerous. Currently, reliable diagnostic criteria are available to make the diagnosis as quick as possible. Treatment is mainly based on corticosteroid therapy in the presence of serious signs. Respecting the rules of prescription remains the most preferable way to prevent avoidable cases.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Keita F, Diatta BA, Coumba N, Deh A, Diop K, Niare N, et al, Diadie S, Niang SO. Profil e?pide?miologique des toxidermies a? Dakar:Etude de 200 cas. Our Dermatol Online. 2022;13(Supp. 2):40-4.
2. Cacoub P, Musette P, Descamps V. The DRESS syndrome:A literature review. Am J Med. 2011;124:588-97.
3. Sayhi S, Achour TB. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome with ciprofloxacin. Our Dermatol Online. 2020;11:189.
4. Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS):An update in 2019. Allergol Int. 2019;68:301-8.
5. Criado PR, Criado RF, Avancini JM, Santi CG. Drug reaction with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome (DIHS):A review of current concepts. An Bras Dermatol. 2012;87:435-49.
6. Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS):An original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-80.
7. Chhiti S, Douhi Z, Alaoui IK, Elloudi S, Baybay H, Mernissi FZ. DRESS syndrome with carbamazepine and Epstein–Barr virus reactivation. Our Dermatol Online. 2023;14:92-4.
8. Lee T, Lee YS, Yoon SY, Kim S, Bae YJ, Kwon HS, et al. Characteristics of liver injury in drug-induced systemic hypersensitivity reactions. J Am Acad Dermatol. 2013;69:407-15.
9. Shanshal M. Dermatologic Emergencies CME Part I:Inflammatory disorders, angioedema, and anaphylaxis. Our Dermatol Online. 2022;13:475-82.
10. Shiohara T, Kano Y. Drug reaction with eosinophilia and systemic symptoms (DRESS):Incidence, pathogenesis and management. Expert Opinion on Drug Safety. 2017;16:139-47.
11. Peyrie?re H, Dereure O, Breton H, Demoly P, Cociglio M, Blayac JP, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms:Does a DRESS syndrome really exist?Br J Dermatol. 2006;155:422-8.
12. Chen YC, Cho YT, Chang CY, Chu CY. Drug reaction with eosinophilia and systemic symptoms:A drug-induced hypersensitivity syndrome with variable clinical features. Dermatol Sin. 2013;31:196-204.
13. Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, et al. The DRESS syndrome:A literature review. Am J Med. 2011;124:588-97.
14. Cho YT, Liau JY, Chang CY, Yang CW, Chen KL, Chen YC, et al. Co-existence of histopathological features is characteristic in drug reaction with eosinophilia and systemic symptoms and correlates with high grades of cutaneous abnormalities. J Eur Acad Dermatol Venereol. 2016;30:2077-84.
15. Eshki M, Allanore L, Musette P, Milpied B, Grange A, Guillaume JC. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms:A cause of unpredictable multiorgan failure. Arch Dermatol. 2009;145:67-72.
16. Cho YT, Yang CW, Chu CY. Drug reaction with eosinophilia and systemic symptoms (DRESS):An interplay among drugs, viruses, and immune system. Int J Mol Sci. 2017;18:1243.
17. Kano Y, Tohyama M, Aihara M, Matsukura S, Watanabe H, Sueki H, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms:Survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42:276-82.
18. Descamps V, Ben Said B, Sassolas B, Truchetet F, Avenel-Audran M, Girardin P. [Management of drug reaction with eosinophilia and systemic symptoms (DRESS)]. Ann Dermatol Venereol. 2010;137:703-8.
19. Funck-Brentano E, Duong TA, Bouvresse S, Bagot M, Wolkenstein P, Roujeau JC. Therapeutic management of DRESS:A retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-52.
20. Ingen-Housz-Oro S, Duong TA, de Prost N, Colin A, Fardet L, Lebrun-Vignes B, et al. [Treatment of severe cutaneous adverse drug reactions]. Ann Dermatol Venereol. 2018;145:454-64.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-8382-8721 http://orcid.org/0000-0002-8382-8721 http://orcid.org/0009-0003-4835-0077 http://orcid.org/0009-0003-4835-0077 http://orcid.org/0009-0001-2247-1781 http://orcid.org/0009-0001-2247-1781 http://orcid.org/0000-0003-0231-1665 http://orcid.org/0000-0003-0231-1665 http://orcid.org/0000-0002-2927-4983 http://orcid.org/0000-0002-2927-4983 http://orcid.org/0000-0003-1885-6563 http://orcid.org/0000-0003-1885-6563 http://orcid.org/0000-0002-5204-0528 http://orcid.org/0000-0002-5204-0528 |






Comments are closed.