Annular and ulcerative lichen planus induced by nivolumab therapy
Tatsuhiko Mori, Toshiyuki Yamamoto
Department of Dermatology, Fukushima Medical University, Fukushima, Japan
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
Sir,
Lichen planus (LP) or lichenoid dermatitis are often observed in patients treated with immune checkpoint inhibitors (ICIs). Herein, we report a case presenting with rare forms of ulcerative as well as annular LP during nivolumab therapy.
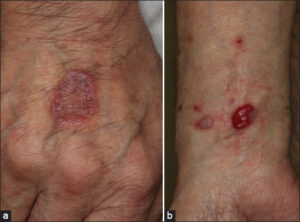
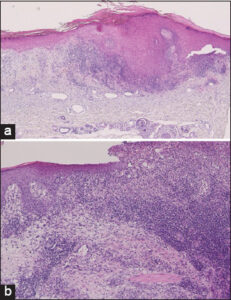
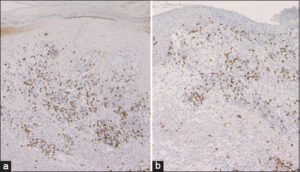
A 73-year-old male began nivolumab (240 mg every two weeks) therapy for unresectable oropharyngeal cancer. One year later, after the twenty-fourth administration of nivolumab, itchy eruption appeared and gradually worsened, and he was referred to our department. A physical examination revealed several well-circumscribed, annular plaques with slightly elevated borders on the dorsum of the hands and forearms (Fig. 1a). Additionally, several keratotic plaques and ulcerated plaques were observed on the upper and lower extremities (Fig. 1b). A biopsy specimen taken from the annular plaque revealed wedge-shaped epidermal acanthosis with focal hyperkeratosis, interface changes with mononuclear cell infiltration of the basement membrane of the epidermis, individual cell keratinization, and cellular infiltration in the upper dermis (Fig. 2a). Another biopsy specimen, taken from the ulcerated plaque, revealed a lack of epidermis and a dense infiltration of mononuclear cells in the upper-to-mid-dermis (Fig. 2b). Infiltrative mononuclear cells were mainly composed of CD8-positive T-cells in both specimens (Figs. 3a and 3b). Nivolumab was continued further for five months; however, due to the tumor growth, the chemotherapy was switched to paclitaxel and cetuximab combination therapy. Thereafter, the skin lesions gradually disappeared after treatment with topical corticosteroid ointment within four months.
LP or LP-like eruptions frequently occur on the trunk and extremities, with a mean time of 6 to 12 weeks after the initiation of ICI therapy [1]. By contrast, our case developed LP nearly one year after therapy initiation. Our case may indicate that LP or LP-like lesions may occur even at a late phase of ICI treatment. In a cohort study at a single institution, lichenoid reactions were observed in 14 of 82 patients (17%) with metastatic melanoma who received anti-PD-1 therapy [2]. Lichen mucosa and nail LP have sometimes been reported. Rare phenotypes, such as annular, hypertrophic, erosive, and bullous LP, have been observed; however, ulcerative LP is extremely rare in ICI therapy [3].
The main pathogenesis of LP is epidermal basal layer damage caused by autoreactive cytotoxic CD8+ T-cells mediated by interferon-γ (IFN-γ). In murine in vivo models of LP, abundant expression of PD-L1 in keratinocytes plays a protective role against cytotoxic CD8+ T-cells [4], which suggests that the blockade of the PD-1/PD-L1 pathway may induce epidermal damage by cytotoxic T-cells. In vitro studies have shown an increased production of IFN-γ from peripheral blood mononuclear cells in patients who developed oral LP after anti-PD-1 antibody administration [5]. Other studies showed elevated mRNA levels of IFN-γ and granzyme B after nivolumab treatment [6], suggesting that such molecules induce interface changes and epidermal damage. Ulcerative changes in the present case may have been attributable to extensive epidermal damage caused by CD8+ T-cells, mediated by IFN-γ, granzyme, and other molecules activated by PD-1 inhibition.
Consent
The examination of the patient was conducted according to the Declaration of Helsinki principles.
REFERENCES
1. Geisler AN, Phillips GS, Barrios DM, Wu J, Leung DYM, Moy AP, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-68.
2. Hwang SJ, Carlos G, Wakade D, Byth K, Kong BY, Chou S, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma:A single-institution cohort. J Am Acad Dermatol. 2016;74:455-61.
3. Niesert AC, Guertler A, Schutti O, Engels L, Flaig M, French LE, et al. Ulcerated lichen planus after adjuvant use of programmed cell death-a-inhibitor:A case report and systematic review of the literature. Acta Derm Venereol. 2021;101:adv00472.
4. Okiyama N, Katz SI. Programmed cell death (PD-1) regulates the effector function of CD8 T cells via PD-L1 expressed on target keratinocytes. J Autoimmun. 2014;53:1-9.
5. Zhou G, Zhang J, Ren XW, Hu JY, Du GF, Xu XY. Increased B7-H1 expression on peripheral blood T cells in oral lichen planus correlated with disease severity. J Clin Immunol. 2012;32:794-801.
6. Anegawa H, Otsuka A, Kaku Y, Nonomura Y, Fujisawa A, Endo Y, et al. Upregulation of granzyme B and interferon-g mRNA in responding lesions by treatment with nivolumab for metastatic melanoma:A case report. J Eur Acad Dermatol Venereol. 2016;30:e231-2.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0001-8771-4206 http://orcid.org/0000-0001-8771-4206 http://orcid.org/0000-0002-8390-2573 http://orcid.org/0000-0002-8390-2573 |




Comments are closed.