Sociodemographic and clinical characteristics of chronic urticaria among patients attending the dermatology clinic of a tertiary-care hospital
Madhu Gyawalee , Vikash Paudel
, Vikash Paudel
Department of Dermatology and Venereology, Patan Academy of Health Sciences, Lagankhel, Lalitpur, Nepal
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Chronic urticaria (CU) is characterized as the recurrent occurrence of wheals, angioedema, or both on most days of the week, for more than six weeks. Information available on this disease is mainly based on foreign studies. We observed the clinical characteristics of this disease among our population to fill the shortage of information.
Materials and Methods: It was a hospital-based, cross-sectional, descriptive study conducted at the Department of Dermatology from July 2022 to March 2023. Patients diagnosed with CU were enrolled in this study after obtaining ethical approval from the Institutional Review Committee (IRC). The calculated sample size was 123. Sociodemographic features and clinical characteristics were recorded after taking consent from the patients. A descriptive analysis was performed and presented in frequency tables.
Results: The majority (61%) had chronic spontaneous urticaria (CSU), 13.8% had chronic inducible urticaria (CINDU), and 25.2% had both CSU and CINDU. The mean age of participants was 35.86 ± 13.45 years. Females comprised 72.4% of the patients. A family history of urticaria was found in 16.2% of patients. The mean disease duration was 35.88 ± 60.2 months. Wheals occurred in the evening in 24.3% of cases. Angioedema was reported by 18.6% of the patients. Gastritis was the most common (11.4%) comorbidity. Physical factors precipitated urticaria in 39% of cases. Recurrence of the disease was seen in 17.8%. Prior to visiting the dermatologist, 76.4% had been taking antihistamines and 15% attempting an alternative medicine.
Conclusion: Our findings were consistent with those of previous reports. CSU is more than three times more common than CINDU. Females and young adults were more affected by CU. Concomitant CSU and CINDU is also possible. As a chronic condition, it is often difficult to manage, and patients tend to explore alternative options.
Key words: Chronic urticaria, Clinical, Demography, Descriptive study
INTRODUCTION
Chronic urticaria (CU) is characterized as the recurrent occurrence of wheals, angioedema, or both on most days of the week for more than six weeks. CU is further divided into chronic spontaneous (no specific eliciting factor involved) urticaria (CSU) and chronic inducible (specific eliciting factor involved, for instance, cold, heat, or pressure) urticaria (CINDU) [1,2]. The prevalence of CU in Asia has shown an increasing trend, with reports of 3.08% in Korea [3], 0.79% in Taiwan [4], and 2.4% in Nepal [5]. It occurs most commonly in females and has a peak age of onset between 20 and 40 years [6]. Wheals have a more generalized distribution among Polish people, in whom CSU and a family history of CSU were twice more common than CINDU [7]. Likewise, 50% of the CUs were associated with angioedema in Brazilians, and stress was the most common aggravating factor [8]. Whereas Asia and the Middle East were found to have more comorbidity associated with CINDU than CSU [9]. Autoantibodies, infectious diseases, thyroid gland disorders, drugs, and numerous more allergens may precipitate CSU. However, the etiology largely varies in different geographical locations [1]. Only several studies from Nepal have focused mainly on the quality of life of patients with urticaria [10] or its association with autologous serum skin tests [11].
In view of the complex nature of CU, we wished to fill the void of the need for more information regarding the clinical characteristics of such a common disease among our own population.
Since CSU is associated with frequent hospital visits causing a burden to patients, families, and the health care system, as itching, wheals, and angioedema are often not sufficiently controlled [1], this study may help to find different clinical aspects of the disease in our own population, thereby helping in proper management.
MATERIALS AND METHODS
This was a hospital-based, observational, cross-sectional study conducted at the Department of Dermatology of the Patan Academy of Health Sciences, Nepal, from July 2022 to March 2023. After obtaining ethical approval from the Institutional Review Committee (IRC), patients diagnosed with chronic urticaria were included in the study. After briefly describing the study, data was collected from those who gave consent to provide information about their disease. The diagnosis of urticaria was clinical, and the required investigations were sent. Pregnant females, lactating mothers, children under fourteen years of age, wheals lasting more than twenty-four hours, acute urticaria, urticarial vasculitis, and mastocytosis were excluded from the study.
Information about the sociodemographic features of the patients and the clinical characteristics of CU was obtained. The confidentiality of the patients was maintained by not recording any data that would identify the patient as an individual (for instance, name, full address, photographs). Regarding occupation, those involved in laborious work (farmers, porters, field workers, etc.) were categorized as manual workers, and those whose work consisted of mostly sitting (secretaries, clerks, managers, etc.) were categorized as table workers. The place of residence was categorized as rural or urban areas according to the administrative division of Nepal. People from the Terai region and living in the river basin were categorized as from hot regions, and people from the hilly region were categorized as from cold regions. For comorbidities, already diagnosed diseases under treatment were recorded. Complete blood count, random blood sugar, serum creatinine level, stool R/E, urine R/E, and TSH (thyroid stimulating hormone) were sent as laboratory investigations to all patients with CU. A consecutive sampling technique was employed; the required calculated sample was 123. A descriptive analysis was performed and presented in frequency tables.
RESULTS
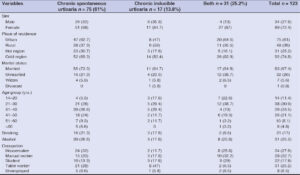
A total of 123 patients with chronic urticaria were included in the study. Out of the 123 patients, a majority (75; 61%) was diagnosed with CSU, while 17 (13.8%) and 31 (25.2%) were diagnosed with CINDU and both (CSU, CINDU), respectively.
The sex distribution revealed that the highest CU was observed among females (89; 72.4%), as compared to 34 (27.6%) males. All types of CU were predominant among females; CSU in 51 (68%), CINDU in 11 (64.7%), and both in 27 (87%) females.
The male-to-female ratio was 1:2.6. The mean age at presentation was 35.86 ± 13.45 years, ranging from 14 to 77 years. The highest number of cases was observed in the age group of 21 to 50 years, and only 4.8% were over 60 years old (Table 1).
In the CSU and CINDU groups, the highest number of cases was observed among the group of 21–40 years, while more cases were observed among the group of 21–30 years in both type groups.
Most of the patients were married (83; 67.4%), living in urban areas (75; 61%), and in cold regions (92; 74.8%) of the country. The majority of the CSU patients (47; 62.7%) were living in urban areas, whereas most of the CINDU patients (9; 53%) were from rural areas of the country. Regarding smoking, 16 (21.3%) of the participants with CSU were smokers, while 20 (26.6%) consumed alcohol.
Twenty-four (32%) of the patients with CSU were housemakers, while 8 (47%) patients with CINDU were table workers. Ten (32.2%) were manual workers in both types of groups.
The mean age at the onset of urticaria was 31.02 ± 13.61 years with an age range of 2 to 73 years. The most common age group for the onset of CU was between 21 and 30 years for all types of CU. The same applied to sex differences.
A family history of urticaria was present in 20 (16.2%) patients with CU. Among these, 15 (20%) of the CSU patients, 1 (5.8%) of the CINDU patients, and 4 (13%) of those with both types of urticaria reported having first-degree relatives with urticaria (Table 2).
The mean disease duration was 35.88 ± 60.2 months, ranging from 1.5 months to 27 years, with patients with longer disease durations being less common.
Thirty-five (46.6%) patients with CSU had the disease for less than one year, and 13 (17.4%) had urticaria for over six years. Among the CINDU patients, 11 (64.7%) had the disease for less than one year, while in only 1 (5.8%) patient, the disease lasted for more than six years.
Out of the total patients, 84 (68.2%) reported daily occurrence of wheals, while for four (3.2%), the appearance of wheals was unpredictable. Everyday occurrence of wheals was primarily reported by patients with both types of urticaria 24 (77.4%) followed by patients with CINDU (12; 70.5%) and CSU (48; 64%).
The mean duration of wheals was 183.3 ± 249.5 minutes, and the duration of wheals ranged from 1 minute to 22 hours. Out of the 123 patients, 39 (31.7%) reported that wheals disappeared within one hour. Nineteen (25.3%) patients with CSU reported that wheals lasted for less than one hour, and in forty-one (54.6%), wheals lasted for 1–6 hours. However, in nine (53%) patients from the CINDU group, the wheals disappeared within one hour, while in seven (41.2%), the wheals lasted for 1–6 hours.
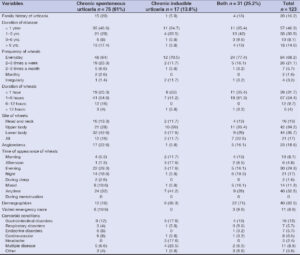
Regarding the distribution of wheals, 86 (70%) patients found wheals either in the upper or lower parts of the body. Seven (5.7%) reported wheals on the scalp, one had wheals on the sole, and twenty-one (17%) had generalized wheals. Thirty-two (42.6%) patients with CSU reported wheals on the lower parts of the body, while ten (59%) patients with CINDU had wheals on the upper parts of the body.
There was no preferential time of day for the occurrence of wheals in forty (32.5%) patients with CU. However, 30 (24.3%) and 21 (17%) reported having wheals during the evening and night, respectively. However, wheals appeared most frequently during the evening among 22 (29.3%) patients with CSU and 3 (17.6%) patients with CINDU. No female patient reported wheals during menstruation.
Urticaria was associated with angioedema in 23 (18.6%) cases, with a higher incidence in females than males (20 vs. 3). Among the 23, 17 (22.6%) had angioedema in the CSU group. The most common site of angioedema was the lips, followed by the eye region. The rest of the patients reported mixed angioedema, affecting the lips, eyes, chin, tongue, or cheeks.
Eleven people (8.9%) were rushed to the emergency department due to generalized urticaria that was uncontrollable with oral antihistamines.
Dermographism was positive in 22 (71%) patients with both types of urticaria, followed by 6 (35.3%) patients with CINDU and 12 (16%) patients with CSU.
Besides urticaria, almost half of the patients (59; 48%) had other preexisting comorbidities. Among these, 16 (13%) diseases were related to the gastrointestinal system, with gastritis being the most common, reported by 14 (11.4%) patients. The other commonly reported comorbidities were hypertension and DM, followed by rhinitis, asthma, migraine, thyroid disorder, hyperlipidemia, dental caries, rheumatoid arthritis, irregular menstruation, and depression.
A routine biochemical test, complete blood count, erythrocyte sedimentation rate, urine, and stool test were performed on all patients on the first visit. Among the 123, 11 (8.9%) patients were found to have other diseases besides the pre-diagnosed disease. Among them, three were incidentally diagnosed with diabetes mellitus after showing high random blood sugar (> 220 mg/dL) and glycosuria. Meanwhile, one patient was found to be anemic (Hb 9.3 g/dL) and two were diagnosed with hypothyroidism. Additionally, four patients had UTIs, and one had hypertriglyceridemia (TG 400).
While most (42.2%) could not associate anything with the occurrence of urticaria, around 39% of the patients reported physical factors (heat, cold, tight clothing, rubbing during baths, exercise) as the precipitating factors for urticaria. One patient experienced wheals every time he sat for studying (Fig. 1).
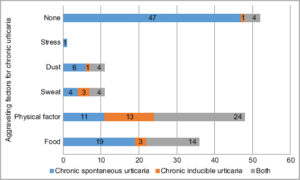 |
Figure 1: Precipitating factors or aggravating factors of chronic urticaria (multiple-response answers). |
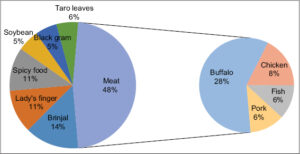
Food was reported as a precipitating factor in 36 (29.2%) patients, with meat (48%) being the most common culprit for 17 (48%) patients, in which buffalo meat precipitated urticaria in ten (28%) patients (Fig. 2).
Recurrence of the disease was seen in 22 (17.8%) patients, with a higher incidence among females (16; 72.7%) than males (6; 27.3%). Most recurrences of urticaria were observed between the age of 21 and 50 years, more commonly in the age group of 21–30 years; among these, 18 (81.8%) patients with CSU had more than one episode of urticaria in their lifetime, and four (18.2%) patients with both types of urticaria had a recurrence of urticaria.
Other symptoms besides itching were reported by 39 (31.7%) patients, a burning and heat sensations being most commonly reported by thirteen patients; cough, throat irritation, and chest discomfort were reported by eleven patients, and dizziness, light-headedness, weakness, yawning, feeling irritated and restless, sleep disturbances were, among others, reported by fifteen patients. Such symptoms were primarily experienced by 26 (34.6%) patients with CSU.
The study found that 110 (89.4%) patients received some form of treatment prior to visiting the hospital, with only 23% continuing their medication. Among the patients who had previously taken medication, 94 (76.4%) had been taking only antihistamines at a therapeutic dose; sixteen (13%) had been multiple medications such as different H1 and H2 antihistamines, oral corticosteroids, montelukast, colchicine, and triple combination creams, and only five (4%) patients had been taking higher than the therapeutic dose of antihistamines. The duration of treatment for urticaria ranged from two days to twenty years, with only 82 (66.6%) patients being able to provide information or documentation about their previous treatment.
Among these, 53 (43%) obtained the medication from local medical shops, whereas 19 (15.4%) attempted alternative medicines such as herbal medicine, traditional healers, and worshipping the Naag god (Table 3).
DISCUSSION
The study included 123 patients diagnosed with CU and aimed to investigate the disease’s various sociodemographic and clinical characteristics. The study found that CU was more common in females than males. The highest number of participants with CSU were females (72.4%), and the highest percentage of participants with CINDU were also females (64.7%), which is consistent with other authors’ findings [6,7]. The male-to-female ratio was found to be 1:2.6, similarly to other study results [8]. However, in an Indian study on CU, more males (66) were affected than females (34), with a male-to-female ratio of 1.9:1 [12]. The actual reason for the sex difference is unknown yet thought to be due to the involvement of an autoimmune component in the occurrence of urticaria, with women being more susceptible than men to autoimmune diseases [13].
The mean age of our patients was 35.86 years, which was a similar finding in other studies [8,14,15]. In our study, the mean duration of the disease was 35.88 ± 60.2 months, ranging from 1.5 months to 27 years, which was quite similar to an Indian study in which the mean duration of CU was 40 ± 40.93 months, and ranged from two months to twenty years [15].
The mean age at the onset of wheels was 31.02 ± 13.61 years, with an age range of 2 to 73 years in our study, similarly to the Indian study [15], yet lower than in a Spanish study in which the mean age at onset was 47.3 ± 16.2 years [16].
Maurer reported that CSU accounted for one-third of all CU cases [17]. Approx. 20% of cases experienced CSU and CINDU concurrently [16]. We also found similar results, with 61% of our patients having CSU, 13.8% having CINDU, and 25.2% having concomitant CSU and CINDU.
A similar proportion of CSU (667; 61.1%) was reported in a multicenter study from Poland, with more cases of CINDU (338; 35.1%) and much fewer of both (41; 3.8%) types of urticaria when compared to our study [7]. While in the U.K., the proportion of different urticarias varied from our study; they reported 217 (56%) patients with CSU, 59 (15%) with CINDU, and 57 (15%) with both CSU and CINDU [17].
Most (61%) of our patients with CU lived in urban areas, and it was found that the population living in urban areas was associated with a higher prevalence of urticaria [14]. A similar finding was observed in which lifestyle and environmental factors such as air pollution and urban living were associated with an increased incidence of allergic diseases among children [18].
In our study, 16 (21.3%) patients with CSU were smokers. No patient reported smoking as a triggering or aggravating factor in our study, unlike in China, where sixteen cases (3.1%) reported smoking-induced wheals [19]. It was interesting to note that smoking correlated with a reduced risk of urticaria when compared with the general population [14,20]. Studies have shown that nicotine modulates mast cell activation and inhibits the synthesis of pro-inflammatory cytokines [21]. However, no causal relationship was found between cigarette and alcohol use in the incidence of CU [8].
Thirty-one (25.2%) patients in our study consumed alcohol, unlike Zhong’s findings in China, where 687 (25.4%) patients consumed alcohol, among whom 383 (55.7%) reported that alcohol triggered their urticaria. Alcohol as an aggravating factor was also reported by Salvaris in Brazil [8]. No patient in our study reported alcohol as a triggering factor for urticaria. There are few case reports of the induction of urticaria after consumption or contact with alcohol [22,23]. Yet, the exact pathogenesis of mast cell activation is not properly understood, whether it is an immunologic or non-immunologic reaction [23].
The observation is that CIU is much more frequent among first-degree relatives of affected individuals than in the general population [24]. In our study, twenty (16.2%) patients with CU reported a family history of urticaria, with CSU reported by fifteen (20%). However, a family history of urticaria varied in different studies; in Nepal, it was found in 24.6% of patients [25], 11.7% in Poland [7], and 4% in Italy. Such familial occurrence of CU suggests the existence of a genetic background for the disease [24].
In our study, more than one episode of CSU was observed in 22 (17.8%) cases, and all had one episode a long time earlier, which was consistent with the study from Spain [16]. Recurrence was observed in 13% of patients with CSU, mainly among alternative medicine users and antihistamine refractory cases [26].
Angioedema is a sudden, pronounced, circumscribed, non-pitting swelling of the deeper dermis and subcutaneous tissue or mucous membranes, presenting as pain or a burning sensation rather than itching [27]. Angioedema may occur with or without urticaria. In up to around 40% of cases, angioedema occurs concurrently with urticaria [13].
We found angioedema concomitant with urticaria in 23 (18.6%) patients, more than in a population-based, Chinese study in which angioedema was found in 6.16% of patients [14]. A higher frequency of urticaria angioedema was reported in patients from Brazil (50.4%) [8].
A systemic review revealed considerable regional differences in the occurrence of angioedema, which seemed more prevalent in Europe and the Americas than in Asia [28].
When the patients with angioedema vs. without angioedema were compared, it was found that angioedema seemed to be underreported and was associated with poor quality of life in terms of daily activities and work performance, with a negative impact on healthcare resource utilization [29].
Although the specific cause of urticaria may not be identified in the individual patient, it is often possible to identify non-specific aggravating factors in chronic urticaria, such as drugs, infections, physical factors, food additives, and stress [30].
In our study, 48% of the participants could recognize the aggravating factor of their urticaria, which was unknown for 52%. In contrast, 79.6% of patients from Spain and 84% from Brazil could tell the worsening factors [8,16]. In our study, physical factors and food were common causes of exacerbating factors, whereas in Curto’s study, NSAIDs and stressful life events were the most common exacerbating factors. While stress, as an exacerbating factor, was reported in 15.2% of patients by Silvares [8], we had only one patient whose urticaria was aggravated by stress, which was, interestingly, the stress of school assignments.
Physical factors (heat, cold, tight clothing, rubbing during baths, exercise) were responsible for the aggravation of urticaria in around 39% of our patients, which was much higher than 10.4% of Silvares’s [8], yet less than the 50% found by Sidbad [31].
We found food as an aggravating factor for CU in 36 (29.2%) patients, similarly to 30% in Juhlin’s findings [32], and higher when compared to Ferrer’s study, in which a food allergy was seen only in 4.8% of patients [33]. Our common urticaria-aggravating food was meat, especially buffalo meat, brinjal, lady’s fingers, black gram, and taro leaves. The types of food-causing allergies are different in different countries; seafood, fish, prawn, crab, peanuts, eggs, and wheat were common food allergens in other Western countries, which is rare in our country. Seafood is generally unavailable to the general people in a landlocked country such as Nepal. This difference in food causing urticaria could also be due to differences in food culture and local beliefs regarding food articles.
In our study, 84 (68.2%) patients reported everyday occurrence of wheal, which was higher than 52% reported by others [8] and was primarily found in patients with both types of urticaria 24 (77.4%), followed by the CINDU (12; 70.5%) and CSU (48; 64%) groups of patients.
The signs and symptoms of CSU may occur spontaneously at any time of the day yet commonly during the evening, among 22 (29.3%) patients with CSU and 3 (17.6%) with CINDU, and at night (1; 5.8%), which was less than in a study from Europe (evening: 34%; night: 23%) [34]. The appearance of wheals during the evening and night may reflect the circadian variation in mast cell activation, which is crucial in developing allergic diseases [34,35].
Some patients with urticaria have only cutaneous symptoms, whereas some patients have systemic symptoms, such as headache, joint pain, and gastrointestinal complaints.
Thirty-nine (31.7%) patients had other symptoms besides itching in our study. Itching and burning sensations were expressed by 33% of our patients, supported (31%) by another study [23].
The frequency of arthralgia, abdominal pain, and fever was reported in the literature yet was non-existent in our study [8,29]. Contrary to our findings, Juhlin reported gastritis as a cause of CU in 44% of cases [32].
In our study, almost half of the patients (59; 48%) had other pre-existing comorbidities besides urticaria. The most common were related to the gastrointestinal system (16; 13%), with gastritis being the most common disease reported (14; 11.4%). The other common comorbidities were hypertension and DM, followed by rhinitis, asthma, migraine, thyroid disorder, hyperlipidemia, and dental caries. However, other studies have reported that thyroid diseases and drug allergies were associated with urticaria [15]. Most of the other studies reported comorbid diseases, atopic diseases such as asthma, rhinitis, psychiatric diseases, type 2 diabetes, and hypertension [36]. It has been reported that the successful treatment of H. pylori infection may result in the remission of urticaria [37].
Most (89.4%) of our patients had been taking some form of treatment before visiting the hospital, which was slightly less than Chu’s finding, in which nearly all patients (99.9%) were on treatment [9], yet similar to other patients from France, Germany, and Spain [16,34]. In our study, 76.4% took only antihistamines, less than in Chu’s finding [9]. In ours, five (4%) had been taking higher doses of antihistamines, which contradicts Chu’s finding, in which 12.4% of patients had been taking a higher dose of antihistamines. The higher rate of prior treatment before visiting the dermatologist in our study was because of the easy availability of antihistamines over the counter from a local medical shop, similarly to Maurer’s finding, in which 78% of the respondents had been taking over-the-counter or prescription medication [34]. People visit the dermatologist only after taking OTC medicine for several weeks and not having their pruritus relieved. Moreover, dermatologists are not easily accessible to the general population who do not live in cities in Nepal. The other medicines, apart from antihistamines, were all prescribed by non-dermatologists or by local pharmacists in our study.
Few patients (7; 5.7%) were put on oral corticosteroids to control urticarial symptoms in our study, which was considered a second-line drug. No patient was treated with omalizumab in our study because the drug is unavailable in Nepal, and most of the general population could not afford it because of its high cost.
CU is a chronic disease in which wheals and itching are not adequately controlled, and patients tend to attempt alternative medicine in search of relief for itching. Almost 15% of our patients went for alternative medicine, mainly to traditional healers (7.5%), followed by worshiping the Naag god (4.8%) and using herbal medicine (3.2%). Whole plants, portions of plants, or single extracted active compounds are all used in phytomedicine [38]. These are widely employed in numerous Asian countries for various pathological conditions such as psychiatric diseases, gastrointestinal disorders, and skin diseases for their anti-inflammatory, anti-allergic, and antioxidant effects. Herbal formulas and single medicinal plants are valid alternatives to antihistaminic drugs in patients with CU, showing improvement in symptomatology and the quality of life of patients [38]. Although gradually on decreasing trend, one of the widely practiced treatment methods for any disease is visiting traditional healers such as Dhaamis and Jhankris, who chant mantras to chase off the offending spirit or to calm down God’s anger, responsible for the deceased ailment, including skin diseases. One of the widespread beliefs in Nepal is that skin diseases result from the Naag god’s anger. Thus, people worship the god and then only visit the hospital or other health facility if they do not feel relieved.
Apart from the aforementioned, a famous Chinese alternative medicine, acupuncture, was found to be effective in up to 90% of cases, reducing the mean duration of disease, suggesting it for the treatment of CU, especially the resistant forms [37].
CONCLUSION
In our study, as in previous studies, females outnumbered males, common in the age group of 21–40 years. Most patients were married homemakers, from urban areas, and from cold climates. A family history was positive in around one-sixth of all participants. Daily occurrence of wheals was the most common. Most urticarial wheals appeared in the evening and night. Angioedema was associated with almost one-fifth of the cases, with the lips being the most common site. Besides itching, nearly a third of the patients experienced other symptoms because of urticaria, commonly burning and heat sensations. The most common precipitating food was meat, especially buffalo meat. Most had been taking antihistamines before coming to a dermatology consultation.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Zuberbier T, Aberer W, Asero R, Latiff A H, Baker D, Balmer-Weber, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2018;73:1393-414.
2. Maurer M, Abuzakouk M, Bérard F, Canonica W, Elberink H O, Arnau A, et al. The burden of chronic spontaneous urticaria is substantial:Real-world evidence from ASSURE-CSU. Allergy. 2017;72:2005-16.
3. Kim BR, Yang S, Choi JW, Choi CW, Youn SW. Epidemiology and comorbidities of patients with chronic urticaria in Korea:A nationwide population-based study. J Dermatol. 2018;45:10-6.
4. Chu CY, Cho YT, Jiang JH, Lin EIC, Tang CH. Epidemiology and comorbidities of patients with chronic urticaria in Taiwan:A nationwide population-based study. J Dermatol Sci. 2017;88:192-8.
5. Shrestha D, Gurung D, Rosdahl I. Prevalence of skin diseases and impact on quality of life in the hilly region of Nepal. J Inst Med Nepal. 2013;34:44-9.
6. Sussman G, Hébert J, Gulliver W, Lynde C, Waserman S, Kanani A, et al. Insights and advances in chronic urticaria:A Canadian perspective. Allergy Asthma Clin Immunol. 2015;11:1-7.
7. Jankowska-Konsur A, Reich A, Szepietowski J. Clinical characteristics and epidemiology of chronic urticaria:A nationwide, multicentre study on 1091 patients. Adv Dermatol Allergol. 2019;36:184-91.
8. Silvares MRC, Coelho KIR, Dalben I, Lastória JC, Abbade LPF. Sociodemographic and clinical characteristics, causal factors and evolution of a group of patients with chronic urticaria-angioedema. Sao Paulo Med J. 2007;125:281-5.
9. Chu CY, Al Hammadi A, Agmon-Levin N, AtKn N, Farag A, Arnaout RK, et al. Clinical characteristics and management of chronic spontaneous urticaria in patients refractory to H1-Antihistamines in Asia, Middle-East and Africa:Results from the AWARE-AMAC study. World Allergy Organ J. 2020;13:100117.
10. Paudel S, Parajuli N, Sharma RP, Dahal S, Paudel S. Chronic urticaria and its impact on the quality of life of Nepalese patients. Dermatol Res Pract. 2020;2020:1-5.
11. Giri U, Kayastha BM, Shakya NB. A hospital-based study of association of chronic spontaneous urticaria with autologous serum skin test. Nepal J Dermatol Venereol Leprol. 2020;18:9-14.
12. Krishna AV, Sunki K, Koneti BB, Amala R, Lavidya A, Harika M. A cross-sectional study on dental infections in chronic urticaria. Int J Res Dermatol. 2020;6:329-32.
13. Fine LM, Bernstein JA. Guideline of chronic urticaria beyond. Allergy Asthma Immunol Res. 2016;8:396-403.
14. Li J, Mao D, Mao D, Liu S, Liu P,Tian j, et al. Epidemiology of urticaria in China:A population-based study. Chin Med J (Engl). 2022;135:1369-75.
15. Mahajan V, Shanker V, Vohra S, Sharma N. Clinicoepidemiologic features of chronic urticaria in patients having positive versus negative autologous serum skin test:A study of 100 Indian patients. Indian J Dermatol Venereol Leprol. 2011;77:156-9.
16. Curto-Barredo L, Archilla L, Vives G, Pujol R, Giménez-Arnau A. Clinical features of chronic spontaneous urticaria that predict disease prognosis and refractoriness to standard treatment. Acta Derm Venereol. 2018;98:641-7.
17. Humphreys, Hunter. The characteristics of urticaria in 390 patients:Urticaria in 390 patients. Br J Dermatol. 1998;138:635-8.
18. Ding G, Ji R, Bao Y. Risk and protective factors for the development of childhood asthma. Paediatr Respir Rev. 2015;16:133-9.
19. Zhong H, Song Z, Chen W, Li H, He L, Gao T, et al. Chronic urticaria in Chinese population:A hospital-based multicenter epidemiological study. Allergy. 2014;69:359-64.
20. Lapi F, Cassano N, Pegoraro V, Cataldo N, Heiman F, Cricelli I, et al. Epidemiology of chronic spontaneous urticaria:Results from a nationwide, population-based study in Italy. Br J Dermatol. 2016;174:996-1004.
21. Mishra NC, Rir-sima-ah J, Boyd RT, Singh SP, Gundavarapu S, Langley RJ, et al. Nicotine inhibits FceRI-induced cysteinyl leukotrienes and cytokine production without affecting mast cell degranulation through a7/a9/a10-nicotinic receptors. J Immunol. 2010;185:588-96.
22. Nakagawa Y, Sumikawa Y, Nakamura T, Itami S, Katayama I, Aoki T. Urticarial reaction caused by ethanol. Allergol Int. 2006;55:411-4.
23. Hadjieconomou S, Mughal A. Segmental urticaria triggered by alcohol consumption. JAAD Case Rep. 2020;6:144-5.
24. Asero R. Chronic idiopathic urticaria:A family study. Ann Allergy Asthma Immunol. 2002;89:195-6.
25. Karki Anupama, Kayastha BM. Chronic Idiopathic Urticaria and its association with antithyroglobulin antibody. Post-Grad Med J NAMS. 2011;11.
26. Kim JK, Har D, Brown LS, Khan DA. Recurrence of chronic urticaria:Incidence and associated factors. J Allergy Clin Immunol Pract. 2018;6:582-5.
27. Memon RJ, Tiwari V. Angioedema. 2023 Jan 14. In:StatPearls [Internet]. Treasure Island (FL):StatPearls Publishing;2023 Jan–. 28. Weerasubpong P, Jiamton S, Phumariyapong P, Ungprasert P, Kulthanan K.
28. Prevalence of concomitant angioedema in chronic spontaneous urticaria:A systematic review and meta-analysis. Asian Pac J Allergy Immunol. 2023;41:12-9.
29. Sussman G, Abuzakouk M, Bérard F, Canonica W, Oude Elberink H, Giménez-Arnau A, et al. Angioedema in chronic spontaneous urticaria is underdiagnosed and has a substantial impact:Analyses from ASSURE-CSU. Allergy. 2018;73:1724-34.
30. Griffiths CEM, Barker J, Bleiker Tanya, Chalmers R, Creamer D. Rook’s Textbook of Dermatology. Ninth. Blackwell Publishing, Ltd;2010.
31. Sibbald RG, Cheema AS, Lozinski A, Tarlo S. Chronic urticaria:Evaluation of the role of physical, immunologic, and other contributory factors. Int J Dermatol. 1991;30:381-6.
32. Juhlin L. Recurrent urticaria:Clinical investigation of 330 patients. Br J Dermatol. 1981;104:369-81.
33. Ferrer M. Epidemiology, healthcare, resources, use and clinical features of different types of urticaria. Alergológica 2005. J Investig Allergol Clin Immunol. 2009;19:21-6.
34. Maurer M, Ortonne JP, Zuberbier T. Chronic urticaria:An internet survey of health behaviors, symptom patterns, and treatment needs in European adult patients. Br J Dermatol. 2009;160:633-41.
35. Nakao A, Nakamura Y. Time will tell about mast cells:Circadian control of mast cell activation. Allergol Int. 2022;71:425-31.
36. Alen Coutinho I, Regateiro FS, Fernandes RA, Pita JS, Gomes R, Coelho C, et al. Refractory chronic urticaria in adults:Clinical characterization and predictors of severity. Allergy Asthma Clin Immunol. 2020;16:97.
37. Iraji F, M Saghayi, H Mokhtari, A Siadat. Acupuncture in the treatment of chronic urticaria:A double-blind study. Internet J Dermatol. 2005;3:1-5.
38. Gammeri L, Panzera C, Calapai F, Cicero N, Gangemi S. Asian herbal medicine and chronic urticaria:Which are the therapeutic perspectives?Nat Prod Res. 2023;37:1917-34.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-5604-8332 http://orcid.org/0000-0002-5604-8332 http://orcid.org/0000-0001-8729-052X http://orcid.org/0000-0001-8729-052X |





Comments are closed.