Skin cancer: Epidemiological, clinical, and histological aspects in albinos and non-albinos in Yaoundé, Cameroon
Coralie Reine Bertine Mendouga Menye , Grâce Anita Nkoro Ombede, Kevin Hervé Lebogo, Emmanuel Armand Kouotou
, Grâce Anita Nkoro Ombede, Kevin Hervé Lebogo, Emmanuel Armand Kouotou
Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Cameroon
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Individuals with phototype VI compared to albinos have a natural protection against the cancerogenic effect of UV rays. The aim of this study was to describe the epidemiological, clinical, and histopathological aspects of skin cancer in albinos and non-albinos.
Methodology: We conducted a descriptive, cross-sectional study over a period of ten years. Data collection was retrospective and exhaustive in five dermatology units and one oncology unit in Yaoundé. Patients having skin cancer and a complete medical file were included in the study. Sociodemographic, epidemiological, clinical, and histopathological variables were studied. Data analysis was performed with SPSS, version 20.0, and Excel 2016.
Results: We included 134 patients. The average age was 43.7 ± 12.2 years, with extremes of 15 and 80 years. The sex ratio among the albinos was 1/1.2, and 1/1 among the non-albinos. The western region was the most represented (44%). The time to diagnosis varied from 3 to 64 months. The face, low neckline, and upper limbs were most frequently involved. The most frequent cancers diagnosed after histopathological confirmation in decreasing order were: epidermoid carcinoma, melanoma, basal cell carcinoma, and Bowen’s disease. Furthermore, the proportion of skin cancer has doubled in ten years.
Conclusion: At the end of our study, we are able to say that skin cancers in Yaoundé are more frequent among albinos.
Key words: Skin cancer; Albino people; Non-albino people; Carcinoma; Yaoundé; Cameroon
BACKGROUND
In Cameroon, little data exists on the clinical, epidemiological, and paraclinical aspects of skin cancer among albinos while albinism is frequent in the western region of Cameroon. This study is a source of data on the subject coming from central Africa.
INTRODUCTION
Albinism is a rare genetic, hereditary, non-contagious affection expressed by a lack of melanin in the skin, hair, and eyes [1]. There are various types of albinism, yet the most common and visible is oculocutaneous albinism, which affects the skin, hair, and eyes [2]. Albinism concerns all races in the world, independently of ethnical group or sex. Albinos are highly vulnerable to skin cancer [3].
Because of the lack of melanin, people affected by albinism are more sensitive to the harmful effects of UV rays, leading to problems such as photophobia, decreased visual acuity, extreme sun sensitiveness, and skin cancer. These noxious effects of UV cause DNA modifications that may lead to anarchic cell multiplication, thus creating cancer [4]. Skin cancer mostly affects the head, and epidermoid carcinoma is more frequent than basal cell carcinoma in the African population, with a ratio of 2:1 among albinos [5,6]. High levels of exposure to UV rays increase the risk of the three main forms of skin cancer [7].
Albinism affects 1 person per 20000 worldwide [8]. Although the incidence of albinism is relatively low in the West, Africa seems to be more affected, with the prevalence fluctuating between 1:15000 and 1:1000 depending on the country and subregion [9,10].
In Cameroon, little data exists on the clinical, epidemiological, and paraclinical aspects of skin cancer among albinos. We, therefore, purposed to conduct a study in Yaoundé, Cameroon, in order to describe the epidemiological, clinical, and histopathological characteristics of skin cancers among albinos and compare these cancers to those among black, non-albino individuals.
METHODS
We conducted a retrospective, descriptive, cross-sectional study from February to March 2021. Patients followed up between January 2011 and December 2020 were concerned (a period of ten years). The study took place in six health facilities in the town of Yaoundé: University Teaching Hospital Yaoundé, Yaoundé Central Hospital, Yaoundé Military Hospital, Elig-Essono Medicalized Healthcare Centre, Yaoundé General Hospital, and Yaoundé Gynaeco-Obstetric and Paediatric Hospital. Each of these health facilities had a dermatology unit and/or an oncology unit.
Study population
All patients having a skin cancer and having consulted during the study period were concerned. We, thus, included: 1) any patient with oculocutaneous albinism and living with a skin cancer; 2) any albino patient received and followed up at one of our study sites irrespective of age, sex, and origin; 3) any albino patient with an existing and exploitable medical file containing at least 80% of the information searched; and 4) any black, non-albino patient with skin cancer.
Patients having cancers with extracutaneous localizations were excluded.
The sampling was consecutive and exhaustive according to the availability of medical files in the record units of the various hospitals requested.
Procedure
In order to conduct this study, we requested and obtained the approval of the Institutional Committee for Ethics and Research (ICER) of the Faculty of Medicine and Biomedical Sciences (FMBS) of the University of Yaoundé I. Since our study was retrospective, we obtained an exemption of informed consent. Then, we obtained administrative authorizations from the head of each health facility involved in our study to recruit patients.
Data collection was performed with a technical data sheet composed of two sections. The first included sociodemographic data (age, sex, domain of activity, region of residence, and native region). The second included clinical characteristics (medical history, duration of symptoms before diagnosis, signs and symptoms at the moment of diagnosis, number of lesions, site of the lesions, type of cancer, the result of the pathological examination). Data was digitized with a preconceived input mask. On the field, we went to the different hospitals concerned by our study and acceded to the records. The patients filling the inclusion criteria had their medical files collected and data was taken according to the structure of the technical data sheet. Anonymity was respected.
Data analysis
The data obtained was digitized and saved with Excel and Word 2016 and analyzed with IBM SPSS, version 20. Quantitative data was represented as means and medians according to the distribution, and qualitative data was represented as numbers and frequencies.
Proportions were compared with the chi-squared test or the Fisher exact test for small samples.
Ethical considerations
We obtained ethical approval from the Institutional Committee for Ethics and Research (ICER) of the faculty. During the study, we respected the fundamental principles of the Helsinki Declaration on human research.
RESULTS
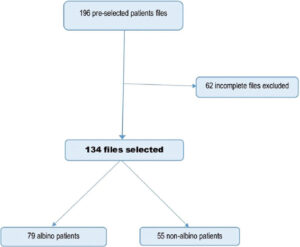
We preselected 196 files for our study, among which 62 (31.6%) were excluded for being incomplete. Among the 134 selected files, 79 were albinos and 55 were non-albinos (Fig. 1). The albino-non-albino ratio was 1.4.
Sociodemographic characteristics
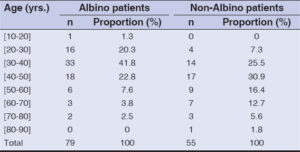
Our sample was mainly made of females (71/134; 53%), with a male-to-female ratio of 0.9. The mean age was 43.7 ± 12.2 years, ranging from 15 to 80 years. The most represented age group was 30 to 40 years (Table 1).
Concerning the sector of activity of our patients, 60.4% were in the private sector (Table 1).
The most frequently found education level was primary and secondary education (Table 1).
The western region was the most represented (59/134; 44%), followed by the southern (24/134; 17.9%) (Table 1).
Epidemiological characteristics
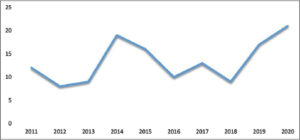
The number of skin cancers has doubled from 12 to 21 cases between 2011 and 2022. The evolution in our study found the first peak in 2014 and the second in 2020 (Fig. 2).
The diagnostic lapse time varied between 3 and 64 months, with an average of 11.7 ± 8.4 months.
Clinical characteristics
Medical and surgical history
In our sample, 15.7% of the patients had undergone at least one surgical excision for skin cancer.
The absence of photoprotection was mainly reported (43.4%). A history of immunosuppression (11.5%), tobacco use (6.6%), and cancer (4.8%) was also reported. Neither arsenic nor hydrocarbon exposure was reported.
Clinical signs
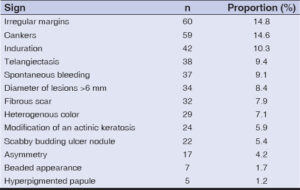
The major clinical presentations observed during the physical examinations were cankers and irregular borders, with respective proportions of 14.6% and 14.8% (Table 2).
Lesion sites
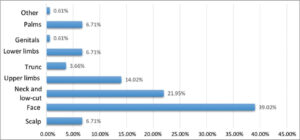
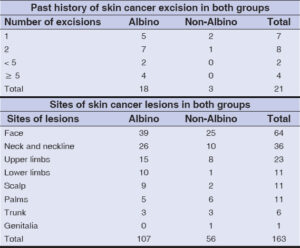
The face and neckline were the most common locations of skin cancers, in 39% and 22% of the cases, respectively (Fig. 3).
The median number of lesions in the sample was 1.7.
Diagnosis of skin cancer
The most frequent diagnosis evoked clinically was epidermoid carcinoma, melanoma, basal cell carcinoma, and Bowen’s disease, with the respective proportions of 61.5%, 23.1%, 13.3%, and 2.1%.
The results of the pathological examination were available in 90 files (67.2%). On histopathology, we found epidermoid carcinoma in 53.3%, melanoma in 36.7%, basal cell carcinoma in 6.7%, and Bowen’s disease in 3.3%.
Comparing results among albinos and non-albinos
Sociodemographic characteristics
In the study, skin cancers were mostly diagnosed in the albinos in the age group of 20–50 years (84.8%), ranging from 15 to 74 years; meanwhile, in the non-albinos, the diagnosis was made ten years later, in the age group of 30–60 years (73.1%), ranging from 26 to 81 years (Table 3).
The western (39/79; 49.4%) and southern regions (16/79; 20.3%) had the most albino patients with cancer as well as non-albinos, among which 36.4% (20/55) originated from the western region and 21.8% (12/55) from the central region.
Comparison of epidemiological characteristics
We noticed two peaks of skin cancers among the albinos: the first in 2015 and the second in 2019. In the non-albinos, three peaks were observed: 2014, 2017, and 2020 (Fig. 4).
The time to diagnosis was between 3 and 64 months among the albinos, with an average of 12.4 ± 9.6 months, while among the non-albinos, the time to diagnosis was between 3 and 36 months, with an average of 10.8 ± 6.3 months.
Comparison of clinical characteristics
A history of skin cancer excision in the albino group was six times more frequent than in the non-albino. The albinos were the only ones to undergo more than two skin cancer excisions. (Table 4).
The albinos had more lesions globally than the non-albinos, ranging from 1 to 22 and from 1 to 3, respectively.
The most frequent sites affected by skin cancer in both albinos and non-albinos were sun-exposed areas (face, neckline, and upper limbs) (Table 4).
Skin cancer diagnosis
The main clinical diagnosis evoked in both groups were epidermoid carcinoma, melanoma, basal cell carcinoma, and Bowen’s disease (Table 5).
The results of histopathology were available in 90 (67.2%) medical files from our sample. More than half (54.2%) of the albino patients had a histopathological diagnosis in their medical file compared to the non-albinos, among whom only 11.8% did not have any.
DISCUSSION
This was a descriptive, retrospective study conducted at different dermatology and oncology units in the town of Yaoundé. The aim of the study was to present the epidemiology, clinical, and histopathological characteristics of skin cancers in Yaoundé among albinos and non-albinos.
The mean age at the occurrence of skin cancer was similar to that in a Malagasy study [11]. These results were similar to those found in the literature, where cancers occurred later in life because of the cumulative effect of UV exposure [12]. A geriatric study conducted by Diabate et al. on the profile of skin pathologies found a mean age of around 72 years; the tumors diagnosed were Kaposi sarcoma, melanoma, and epidermoid carcinoma [13]. Akakpo et al. found a mean age of 52 years in a population on cancer treatment [14].
The male-to-female ratio in our study was 0.9, which was comparable to the sex ratio found by Okafor et al. [15]. Otherwise, the Malagasy study found a slight male predominance [11]. Akakpo et al. found a sex ratio of 0.35 [14].
In our study, the albino-non-albino ratio of cancer occurrence was 1.5/1. This result was opposite to a study conducted in Nigeria by Okafor et al., who found more cases of skin cancers in non-albinos than in albinos [15].
Concerning the native region, the predominance of our cases originating from the western region could be explained by the fact that albinism which, is a risk factor of skin cancer, is more frequent in this region of Cameroon [16].
The incidence of skin cancers doubled from 2011 to 2020. This evolution is comparable to the observations made in Madagascar, where the number of cases increased fourfold between 2008 and 2015 [11]. This increase in incidence could be explained by the improvement in access to healthcare services permitting the confirmation and management of such cases.
The time to diagnosis was highly variable in our study, similarly to the results by Kiprono et al. found in Tanzania, with the time to diagnosis falling between 2 and 56 months; the average time was 26.2 ± 10.2 months [17]. The time before consultation, according to Akakpo et al., varied between 6 and 12 months [14]. The main reasons that could explain this delay were financial challenges and being managed in hospitals far away from adequate healthcare facilities [17]. Since our study was retrospective, we were unable to find the reasons of such a long lapse. However, the reasons found in Tanzania could be applicable in Cameroon, given the access to health facilities, the qualified healthcare provider for diagnosis, and financial challenges.
The descending order of skin lesions seen in skin cancer was similar to that found by Okafor and Malalaniaina [11,15]. This could be explained by the fact that the face is the most sun-exposed part of the body and is the least protected when compared to the neckline and the scalp.
Almost one-third of the patients in our study did not have a histopathological examination available in their medical file. This could be due to financial difficulties found in our environment. Otherwise, the clinical diagnosis easily made by some dermatologists permitted the initiation of treatment without histopathological examination. This assertion is supported by the fact that, in our study, the clinical diagnosis was confirmed by pathology in 98% of the cases. At the same time, we also found fewer histopathology reports in the albinos’ medical files. This could suggest that cancer diagnosis is easier to reach at the clinical stage for these patients.
By order of frequency, the most frequently diagnosed cancers were epidermoid carcinoma, melanoma, and basal cell carcinoma. This result was similar to the results by the Malagasy and Nigerian studies [11,15]. In Western countries, especially in France, the most frequent skin cancers were basal cell carcinoma (70%), epidermoid carcinoma (20%), and melanoma (10%) [18].
The mean age at which skin cancer was diagnosed in the albinos was 33.7 ± 9.2 years in our study, which corroborates the findings made in Tanzania by Kiprono et al. [17]. Among the non-albino patients, the mean age was 48.6 ± 11.7 years, which was four years less than the age found in the non-albino population in a study conducted by Okafor et al. [15].
As in a study by Saka et al. in Togo [19], our study revealed that some patients had more than one lesion.
Study limitations
Our study had some limitations: 1) the lack of digitalized records made access to medical files difficult; 2) the non-inclusion of a number of incomplete files, thus reducing our sample; and 3) missing information in the medical files, leading to difficulties in describing the pathologies.
CONCLUSION
At the end of our study, we could say that skin cancers are frequent in clinics of Yaoundé. Albino patients seem more prone to develop these cancers. Skin cancer affects young people and is found in the majority of people originating from the western region. The incidence of skin cancer has almost doubled in a decade. In our study, skin cancers by order of frequency were epidermoid carcinoma, melanoma, basal cell cancer, and Bowen’s disease. Although the diagnosis was reached at the clinical step in most cases, a histopathological confirmation was necessary to optimize the management of the patients. Photoprotection in albinos and non-albinos remains the most effective and most accessible preventive measure against skin cancer.
Statement of human and animal rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of informed consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. United Nations. Potection des droits humains des personnes atteintes d’albinisme. Genève;2020 juill p. 4. Report No.:A/75/170.
2. Mcbride GR. Oculocutaneous albinism:An African perspective. Br Ir Orthopt J. 2014;11:3 8.
3. Ikponwosa ero. HCDH |Experte indépendante sur l’exercice des droits de l’homme par les personnes atteintes d’albinisme. https://www.ohchr.org/FR/Issues/Albinism/Pages/IEAlbinism.aspx
4. Hong ES, Zeeb H, Repacholi MH. Albinism in Africa as a public health issue. BMC Public Health. 2006;6:212.
5. Yakubu A, Mabogunje OA. Skin cancer in Zaria, Nigeria. Trop Doct. 1995;25 Suppl 1:63 7.
6. Kromberg JG, Castle D, Zwane EM, Jenkins T. Albinism and skin cancer in Southern Africa. Clin Genet. 1989;36:43 52.
7. Glanz K, Saraiya M. Using evidence-based community and behavioral interventions to prevent skin cancer:Opportunities and challenges for public health practice. Prev Chronic Dis. 2005;2:A03.
8. David CV. Oculocutaneous albinism. Cutis. 2013;91:E1-4.
9. Nations U. Journée internationale de sensibilisation àl’albinisme |Nations Unies [Internet]. United Nations. United Nations;2019. https://www.un.org/fr/observances/albinism-day.
10. Okoro AN. Albinism in Nigeria:A clinical and social study. Br J Dermatol. 1975;92:485 92.
11. Malalaniaina A, Lovasoa T, Mamisoa RI, Malala R, Soavina RL, Florine R, et al. [Skin cancers in Madagascar:Where do we stand?]. Pan Afr Med J. 2019;34:167.
12. Roy S. Impact of UV radiation on genome stability and human health. Adv Exp Med Biol. 2017;996:207 19.
13. DiabatéA, Kourouma HS, Vagamon B, GuéI, Kaloga M, Aka BR. Skin pathology of the elderly patients:Case of black African. Our Dermatol Online. 2018;9:19-21.
14. Akakpo AS, Ablavi Adani-Ifi AO, Téclessou JN, Kassang P, Mouhari-Toure A, KombatéK, et al. [Skin and mucosal manifestations of cancer treatments in Lomé(Togo):A series of 35 cases]. Our Dermatol Online. 2022;13(Supp. 1):7-14.
15. Okafor OC, Onyishi NT. Primary cutaneous malignancies in nonalbino and albino Africans. Int J Dermatol. 2021;60:222 8.
16. Aquaron R. Oculocutaneous albinism in Cameroon:A 15-year follow-up study. Ophthalmic Paediatr Genet. 1990;11:255 63.
17. Kiprono SK, Chaula BM, Beltraminelli H. Histological review of skin cancers in African Albinos:A 10-year retrospective review. BMC Cancer. 2014;14:157.
18. Gordon R. Skin cancer:An overview of epidemiology and risk factors. Semin Oncol Nurs. 2013;29:160 9.
19. Saka B, Akakpo SA, Teclessou JN, Gnossike P, Adam S, Mahamadou G, et al. Skin cancers in people with albinism in Togo in 2019:Results of two rounds of national mobile skin care clinics. BMC Cancer. 2021;21:26.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-3879-2659 http://orcid.org/0000-0003-3879-2659 |







Comments are closed.