Clinical and dermatoscopic correlation in facial melanosis: A cross-sectional study at a tertiary hospital
Vijeta Prasad, Alpana Mohta , Rajesh Dutt Mehta, Bhikamchand Ghiya, Prasoon Soni, Sumiti Pareek
, Rajesh Dutt Mehta, Bhikamchand Ghiya, Prasoon Soni, Sumiti Pareek
Department of Dermatology, Venereology and Leprology, Sardar Patel Medical College, Bikaner, Rajasthan
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Facial pigmentary disorders are a group of heterogeneous entities, sharing a common clinical feature of altered pigmentation of the face. The overlapping features among the different clinical entities of facial hyperpigmentation may be differentiated by dermatoscopy.
Objective: The objective was to evaluate the different types of facial melanosis and their dermatoscopic findings.
Methods: This non-interventional, cross-sectional study was conducted at our dermatology department over a period of one year. All clinically diagnosed cases of facial melanosis were included in our study and their dermatoscopic evaluation was performed.
Results: We included 500 patients with facial melanosis. While 370 patients were female, 130 were males. Three hundred patients had melasma, 59 (lichen planus pigmentosus) had LPP, 40 had Riehl’s melanosis, while 35 had frictional melanosis. Other dermatoses included acanthosis nigricans, ashy dermatosis, and poikiloderma of Civatte, to name a few. The most common dermatoscopic finding in our study evident in almost all cases of facial melanosis was exaggerated pseudo-reticular hyperpigmentation. The unique observation in our study, the reticulo-globular pattern seen in melasma, was thin and in the shade of brown pigmentation, whereas in LPP and ashy dermatosis, it was comparatively thicker and grayish-brown in color. The other specific finding observed was telangiectasia due to topical steroids, more prominent and enlarged than telangiectasia due to sun exposure in melasma and poikiloderma of Civatte.
Conclusion: This study highlights the clinical and dermatoscopic features of facial melanosis. Further research is required in this field of dermatoscopic evaluation of facial melanosis to define more accurate diagnostic features as a facial biopsy is mostly refused by the patients, yet meanwhile, clinical evaluation should not be neglected.
Study Limitations: The limitations of our study were the lack of direct histopathological correlation and the inclusion of both therapy-naive patients and patients on therapy.
Key words: Facial melanosis; Dermatoscope; Melasma
INTRODUCTION
Facial melanosis is an umbrella term encompassing various disorders causing hyperpigmentation of the face. All these pigmentary disorders pose a diagnostic challenge to dermatologists as their clinical appearance shows tremendous similarities. Hyperpigmentary disorders either result from an increase in the number of melanocytes or in the activity of melanocytes, and thus excess melanin production. The excess pigment may be epidermal or dermal in location, and in both cases, it may be localized or diffuse. The epidermal pigment appears brownish and results from increased melanin production or increased melanosomal transfer in keratinocytes. The dermal pigment results from the dropping of melanin from the basal cell layer into the dermis (dermal pigment incontinence) and appears bluish or bluish-gray due to the Tyndall effect. Dermoscopy is a handy tool employed for the past several years to inspect various dermatological diseases, among which pigmentary disorders have been at the top of the list.
The dermatoscopic approach to facial pigmentary disorder helps in understanding different patterns of facial pigmentation and their depth of pigmentation. Thus, correlating dermatoscopic features with clinical findings helps in the better understanding of facial pigmentary disorders. This valuable knowledge may be further applied to predict prognosis and treatment outcomes. Our study explored the various dermatoscopic features of facial melanoses.
PATIENTS AND METHODS
This was a non-interventional, cross-sectional study conducted between January 2020 to December 2021 at the dermatology and venereology department in Bikaner, Rajasthan, India, after obtaining approval from the institutional ethical committee. All newly diagnosed cases of facial melanosis were included in our study. Written consent was taken from the individual patients to include them in the study. Post-inflammatory hyperpigmentation was excluded due to vastly different etiologies of little significance. All new patients with facial melanosis irrespective of their age, sex, and previous treatment reported at the OPD were diagnosed after relevant history taking and clinical examination. Dermatoscopic findings of facial melanosis were noted with a handheld dermatoscope (DermLite, 3rd generation, 10× magnification). The digital images were captured with a mobile camera. The images were studied on the computer screen and after analyzing all the images, the interpretation of the dermatoscopic findings was made and recorded. The interpretation of the dermatoscopy was based on patterns described in the literature.
RESULTS
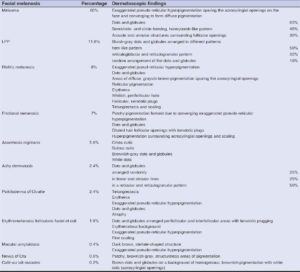
In our study, we examined 500 patients with facial melanosis. The most common facial melanosis was melasma. Out of the 500 patients, 370 (74%) were female and 130 (26%) were male. Out of the 500 patients, there were 300 cases of melasma (60%), 59 of LPP (11.8%), 40 of Riehl’s melanosis (8%), 35 of frictional melanosis (7%), 28 of acanthosis nigricans (5.6%), 12 of ashy dermatosis (2.4%), 12 of poikiloderma of Civatte (2.4%), 8 of EFFC (erythromelanosis follicularis et coli) (1.6%), 2 of macular amyloidosis (0.4%), 3 of nevus of Ota (0.6%), and 1 of café-au-lait macules (0.2%). The most common age group of patients with facial melanosis was 20–30 years of age (51.6%), followed by 30–40 years (35.20%), 40–50 years (6.40%), 12–20 (6%), and 50–60 years (0.8%).
In our study, out of the 300 (60%) melasma patients, 180 (60%) were females and 120 (40%) were males; among the 59 LPP patients, 13 (22.7%) were males and 46 (77.3%) were females; among the 40 patients with Riehl’s melanosis, 14 (35%) were males and 26 (65%) were females; among the 35 cases of frictional melanosis, 32 (91.7%) were males and 3 (8.3%) were females; among the 28 cases of acanthosis nigricans, 16 (57.1%) were males and 12 (42.9%) were females; among the 12 cases of ashy dermatosis, 8 (67%) were females and 4 (33%) were males; among the 8 cases of EFFC, 4 (50%) were males and 4 (50%) were females; among the 12 cases of poikiloderma of Civatte, 10 (83%) were females and 2 (17%) were males; the rest were 1 male and 1 female with macular amyloidosis on the face, 1 male and 2 females with nevi of Ota, and 1 female with café-au-lait macules. The major causes of facial melanosis such as melasma, LPP, and Riehl’s melanosis had a predominance of female patients, whereas frictional melanosis was found more commonly in males.
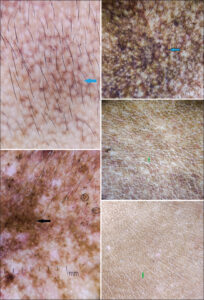
In our study, the most common facial melanosis reported was melasma. The pattern of distribution of melasma observed was malar (n = 240; 80%), followed by centrofacial (n = 54; 18%), and mandibular (n = 6; 2%). Clinically, epidermal melasma patients have light brown pigmentation and deep dermal melasma patients have more dark brown to grayish pigmentation. The most common dermatoscopic finding in melasma (n = 300; 60%), which was evident in almost all cases, was exaggerated pseudo-reticular hyperpigmentation, sparing acrosyringial openings (n = 300; 100%), converging at places to form diffuse pigmentation (Fig. 1). This basic exaggerated pseudo-reticular hyperpigmentation was superimposed by dots and globules (n = 195; 65%) (Fig. 1), a semicircle- and circle-forming, honeycomb-like pattern (n = 135; 45%) (Fig. 1), arcuate and annular structures surrounding follicular openings (n = 90; 30%) (Fig. 1), and a reticuloglobular pattern (n = 240; 80%) (Fig. 1). The other findings were telangiectasia (n = 150; 50%) (Figs. 1 and 2) and atrophy (n = 98; 32.6%) (Fig. 2). Out of the 300 melasma patients, 30 (10%) had dermatoscopic features of exogenous ochronosis, which were brown, amorphous, curvilinear worm-like structures obliterating follicular openings (n = 27;90%) and archiform structures (n = 3; 10%) (Fig. 2).
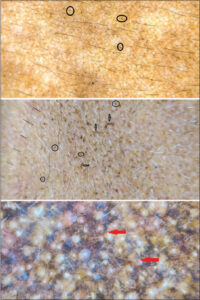
The characteristic dermatoscopic pattern of LPP (n = 59; 11.8%) observed was dark brown to bluish-gray dots and globules arranged in different patterns. The most common pattern was the hem-like pattern (50%) (Fig. 3), followed by the reticulogranular and reticuloglobular pattern (32%) (Fig. 3), and a random arrangement of dots and globules (18%) (Fig. 3). The other findings were the exaggerated pseudo-reticular pattern (Fig. 3) and the accentuation of pigmentation around follicular openings (20%) (Fig. 3).
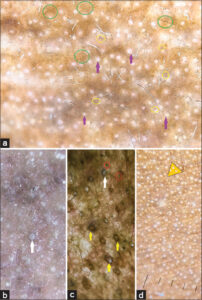
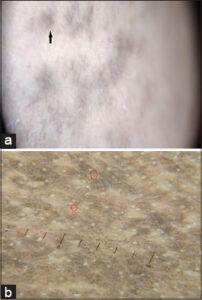
In Riehl’s melanosis (n = 42; 8.4%), we observed dermatoscopic findings of exaggerated pseudo-reticular hyperpigmentation, patchy, diffuse pigmentation, dots and globules, white, fine scales, perifollicular halos, and follicular, keratotic plugs (Fig. 4a).
In all cases of frictional melanosis (n = 35; 7%), the common dermatoscopic findings observed were dilated follicular openings, keratotic plugs, dots and globules, perifollicular hyperpigmentation, and exaggerated pseudo-reticular hyperpigmentation (Figs. 4b and 4c).
The dermatoscopic findings of acanthosis nigricans (n = 28; 5.6%) were sulcus cutis, crista cutis, and brown dots present in crista cutis on a grayish-brown background (Fig. 4d). These similar findings were more accentuated on the neck than on the face.
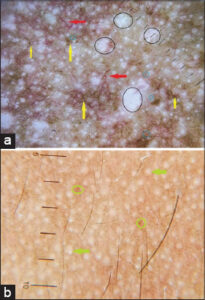
In ashy dermatosis (n = 12; 2.4%), the dermatoscopic findings were exaggerated pseudo-reticular hyperpigmentation, erythema, and grayish-brown dots and globules arranged in an irregular fashion (n = 3; 25%) (Fig. 5) to form linear and circular lines (n = 3; 25%) (Fig. 5), also seen arranged in a reticular pattern to form a reticulogranular or reticuloglobular pattern (n = 6; 50%) (Fig. 5).
The dermatoscopic findings in poikiloderma of Civatte (n = 12; 2.4%) were telangiectasia, erythema, exaggerated pseudo-reticular hyperpigmentation, dots and globules, and atrophy (Fig. 6a).
The dermatoscopic findings in EFFC (n = 8; 1.6%) were follicular keratotic plugging with dots and globules arranged around follicles and in interfollicular areas with background erythema and exaggerated pseudo-reticular hyperpigmentation (Fig. 6b).
Two cases of macular amyloidosis (n = 2; 0.4%) of 1 male and 1 female were included in our study, with the dermatoscopic examination revealing a dark brown, stellate-shaped structure on a background of exaggerated pseudo-reticular hyperpigmentation (Fig. 7).
 |
Figure 7: (macular amyloidosis): White arrow: brown, stellate structure; yellow circle: exaggerated pseudo-reticular hyperpigmentation; black circle: dots and globules. |
The dermatoscopic findings of nevus of Ota (n = 3; 0.6%) were structureless areas of brownish-gray pigmentation (Fig. 8a).
One case of café-au-lait macules (n = 1; 0.2%) on the face was included in our study. The dermatoscopic features observed were the background of brownish hyperpigmentation superimposed by grayish-brown dots and globules and white dots representing acrosyringial openings (Fig. 8b).
DISCUSSION
Facial pigmentary disorders are a group of heterogeneous entities, sharing the common clinical feature of altered pigmentation of the face, causing easily visible cosmetic disfigurement, therefore resulting in significant psychosocial consequences. There is a considerable overlap in features among the different clinical entities of facial hyperpigmentation creating confusion in clinical diagnosis. It is a wonderful fact that more than 80% of the global population has the heterogeneity of facial skin color irrespective of sex and age [1]. Individuals with darker skin are particularly more susceptible to pigmentary disorders as suggested by some studies [2–5]. The reason behind this is that darker individuals have a greater tendency to develop post-inflammatory hyperpigmentation [6]. The other factors influencing our skin pigmentation are endocrine factors, genetic factors, and external agents such as light and photodynamic chemicals. The location of melanin in different layers of skin determines the color; melanin in the upper epidermis (stratum corneum and spinosum) as black, in the dermal–epidermal junction as light to dark brown; in the papillary dermis as slate blue, and in the reticular dermis as steel blue.
Dermoscopy is an important diagnostic tool for observing subtle features to make a diagnosis with precision. Dermoscopic findings also help in correlating underlying histopathological features [7]. Dermatoscopy has been in use for the past several years for the examination of dermatological diseases, for instance, pigmentary disorders. The pigment network lines seen on dermatoscopy are due to pigmentation along the rete ridges, which are thick with a greater quantity of melanin, while the holes in this network are due to relatively less pigmented tips of the dermal papillae with a thin suprapapillary plate [8]. This conventional pigment network is rare when it comes to fairer skin as the rete ridges are not long enough to create a pigment network. The rete ridges on the face rather than in non-facial skin are usually flattened, resulting in the absence of a pigment network, and are accordingly replaced by a pseudo-network pattern in which holes correspond to the adnexal openings of a hair follicle, sebaceous gland, and sweat gland. The dermatoscopic diagnosis of pigmented skin lesions accounts for both local and global features. Global features define patterns while local features cause minor changes.
We conducted our study at the dermatology department of our institution after taking approval from the institutional ethics committee. The study population was all newly diagnosed cases of facial melanosis attending our patient department of dermatology. The examination of the cases was performed after informed consent and the relevant history were taken. The most common facial melanosis seen in our study was melasma (n = 300; 60%). It was found more common in females (n = 180; 60%) than in males (n = 120; 30%). Clinically, the pattern of distribution observed was malar (n = 240; 80%), centrofacial (n = 54; 18%), and mandibular (n = 6; 2%). The basic global dermatoscopic finding observed in melasma was exaggerated pseudo-reticular hyperpigmentation sparing the acrosyringial openings on the face and converging to form diffuse pigmentation (Figs. 1 and 2). This basic exaggerated pseudo-reticular network in melasma was seen superimposed by dots and globules (n = 195; 65%) (Fig. 1), a semicircle- and circle-forming, honeycomb-like pattern (n = 135; 45%) (Fig. 1), arcuate and annular structures surrounding follicular openings (n = 90; 30%) (Fig. 1), and a reticuloglobular pattern (n = 240; 80%) (Fig. 1). This reticular pattern in melasma has already been extensively described in the literature. Sonthalia et al. reviewed the dermatoscopic findings of melasma and concluded that dots, globules, and arcuate and annular structures were the dermatoscopic features of melasma [9]. Nanjundaswamy et al. reported a reticuloglobular pattern in melasma [10]. Yalamanchili et al., on the other hand, described the dermoscopic features of melasma in comparison to other facial melanoses [11]. The different shades of the color of melanin in melasma were suggestive of the depth of pigmentation. The other occasional finding was telangiectasis (n = 150; 50%) (Figs. 1 and 2) due to sun exposure (Fig. 1) and steroid use (Fig. 2). The telangiectasia due to steroids was more prominent and enlarged than due to sun exposure. Dermatoscopy also helped in appreciating other complications such as atrophy (n = 98; 32.6%) (Fig. 2), depigmentation and exogenous ochronosis (n = 30; 10%), dark brown, worm-like pigmentation (n = 27), and arciform structures (n = 3) (Fig. 2). Hence, dermatoscopy also proved to have a prognostic value.
The second most common facial melanosis in our study was LPP (n = 59; 11.8%), the dermatoscopic features were characterized by dots and globules arranged in different patterns. The most common patterns were the hem-like pattern (50%) (Fig. 3), followed by the reticuloglobular and reticulogranular pattern (32%) (Fig. 3), and a random arrangement of dots and globules (18%) (Fig. 3). The other findings were an exaggerated pseudo-reticular pattern and the accentuation of pigmentation around follicular openings (Fig. 3). Neema et al. reported similar findings of the hem-like pattern of pigmentation, perifollicular accentuation, and gray dot and globules [12].
Riehl’s melanosis (n = 42; 8.4%) was the third most common facial melanosis in our study. The dermatoscopic findings (Fig. 4a) were characterized by exaggerated pseud-reticular hyperpigmentation, dots and globules, areas of diffuse, grayish-brown pigmentation sparing the acrosyringial openings, reticular pigmentation, erythema, whitish, perifollicular halo, follicular keratotic plugs, telangiectasia, and mild scaling. Dharman et al. reviewed similar dermatoscopic findings in Riehl’s melanosis [13]. Wang et al. described the same dermatoscopic findings along with clinical, confocal, and histopathological reviews in Riehl’s melanosis [14].
Frictional melanosis (n = 35; 7%) unlike other facial melanoses was more common in males than females in the middle-age group. The dermatoscopic findings (Figs. 4b and 4c) observed were patchy pigmentation formed due to converging exaggerated pseudo-reticular hyperpigmentation, dots and globules, dilated hair follicular openings with keratotic plugs, hyperpigmentation surrounding acrosyringial openings, and scaling. Mutalik et al. described similar dermatoscopic findings in facial frictional melanosis [15].
In acanthosis nigricans (n = 28; 5.6%), the facial dermatoscopic findings (Fig. 4d) were less pronounced than on the neck. The characteristic findings were crista cutis, sulcus cutis, brownish-gray dots and globules, and white dots (Fig. 4d). Crista cutis and sulcus cutis were more readily visible in the non-polarized mode whereas dots and globules were better visible in the polarized mode. Ankad et al. delineated similar findings in the dermatoscopy of acanthosis nigricans [16].
Ashy dermatosis (n = 12; 2.4%) was clinically seen as blue to gray, macular lesions. The main dermatoscopic findings observed were dots and globules on a background of erythematous, diffuse, and brownish pigmentation. Dots and globules were arranged randomly (n = 3; 25%) (Fig. 5), in linear and circular lines (n = 3; 25%) (Fig. 5), in a reticular and reticulogranular pattern (n = 6; 50%) (Fig. 5). Elmas et al. reported similar findings [17].
The unique observation in our study was that the reticuloglobular pattern seen in melasma was thin and in the shade of brown pigmentation, whereas in LPP and ashy dermatosis, it was comparatively thicker and grayish-brown. The hem-like pattern was seen characteristically in LPP, whereas the reticulogranular and reticuloglobular pattern was seen in both LPP and ashy dermatosis.
The dermatoscopic findings seen in poikiloderma of Civatte (n = 12; 2.4%) were telangiectasia, erythema, exaggerated pseudo-reticular hyperpigmentation, dots and globules, and atrophy (Fig. 6a). Sinha et al. reported similar features [18].
In erythromelanosis follicularis faciei et colli (n = 8; 1.6%), the dermatoscopic findings (Fig. 6b) were dots and globules arranged in perifollicular and interfollicular areas with keratotic plugging on an erythematous background with exaggerated pseudo-reticular hyperpigmentation along and fine scaling. Maouni et al. reported the same findings [19].
In the two cases of macular amyloidosis (n = 2; 0.4%) of 1 male and 1 female, dermatoscopic examination revealed a dark brown, stellate-shaped structure on the background of exaggerated pseudo-reticular hyperpigmentation (Fig. 7). Sonthalia et al. described a dermoscopic pattern of macular amyloidosis as a hub and spoke pattern [20].
In nevus of Ota (n = 3; 0.6%), we observed patchy, brownish-gray, structureless areas of pigmentation (Fig. 8a). Vinay et al. revealed the same dermatoscopic features [21].
One case of café-au-lait macules on the face was examined by the dermatoscope. The features seen were brown dots and globules on a homogenous, brownish, pigmented background with white dots (acrosyringial openings) (Fig. 8b).
CONCLUSION
Facial pigmentary disorders are one of the most challenging cases that the dermatologist encounters in their practice and it has profound psychosocial implications in the patient’s life. Although a clinical examination has paramountcy in diagnosing facial melanosis, dermatoscopy has also valuable input in diagnosing facial pigmentary disorder as a biopsy from the face is often refused by the patient. By evaluating the depth of pigmentation by the dermatoscope, further inference over prognosis and treatment outcome may be predicted more efficiently. Hence, studies exploring this particular dermatoscopic examination domain are of great significance.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Hourblin V, Nouveau S, Roy N, De Lacharriere O. Skin complexion and pigmentary disorders in facial skin of 1204 women in 4 Indian cities. Indian J Dermatol Venereol Leprol 2014;80:395-401.
2. Halder RM, Grimes PE, McLaurin CI, Kress MA, Kenney JA Jr. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388-90.
3. Sanchez MR. Cutaneous diseases in Latinos. Dermatol Clin. 2003;21:689-97.
4. Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders of skin of color:A comparative practice survey. Cutis. 2007;80:387-94.
5. Grimes PE. Management of hyperpigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28:77-85.
6. Sandhu S, Neema S, Radhakrishnan S. Dermoscopy of disorders of hyperpigmentation. Pigment Int. 2021;8:14-24.
7. Argenziano G, Soyer HP. Dermoscopy of pigmented skin lesions:A valuable tool for early diagnosis of melanoma. Lancet Oncol. 2001;2:443-9.
8. Adya KA, Inamadar AC, Palit A. Dermoscopic pigment network:Characteristics in non-melanocytic disorders. Indian Dermatol Online J. 2020;11:146-5.
9. Sonthalia S, Jha AK, Langar S. Dermoscopy of melasma. Indian Dermatol Online J. 2017;8:525-6.
10. Nanjundaswamy BL, Joseph JM, Raghavendra KR. A clinico-dermoscopic study of melasma in a tertiary care center. Pigment Int. 2017;4:98-103.
11. Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
12. Neema S, Jha A. Lichen planus pigmentosus. Pigment Int. 2017;4:48-9.
13. Dharman BK, Sridhar S. Diffuse facial melanosis:An overview of etiology and dermoscopic findings. J Skin Sex Transm Dis. 2020;2:86-93.
14. Wang L, Xu AE. Four views of Riehl’s melanosis:Clinical appearance, dermoscopy, confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2014;28:1199-206.
15. Mutalik SD, Pethe SV, Nikam BP, Rasal YD. Facial frictional melanosis in Indian patients:Defining the entity. Clin Dermatol Rev. 2019;3:78-83.
16. Ankad BS, Drago NR, Koti VR, Nikam BP. Dermoscopic approach to hyperpigmented lesions in skin of color. Clin Dermatol Rev. 2020;4:84-91.
17. Elmas &F, Acar EM, Kilitçi A. Dermoscopic diagnosis of ashy dermatosis:A retrospective study. Indian Dermatol Online J. 2019;10:639-43.
18. Sinha P, Tripathy DM, Shelly D, Neema SA rare case of poikilodermatous mycosis fungoides. Indian J Dermatol. 2020;65:417-9.
19. Maouni S, El Anzi O, Sqalli A, Znati K, Meziane M, Hassam B. Erythromelanosis follicularis faciei et colli:Dermoscopy and dermatopathology correlates. JAAD Case Rep. 2019;5:535-6.
20. Sonthalia S, Agrawal M, Sehgal VN. Dermoscopy of macular amyloidosis. Indian Dermatol Online J. 2021;12:203-5.
21. Vinay K, Ankad BS. Dermatoscopic features of pigmentary diseases in ethnic skin. Indian Dermatol Online J. 2021;12:24-33.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0001-7526-2089 http://orcid.org/0000-0001-7526-2089 |






Comments are closed.