Febrile diseases in patients hospitalized at the Department of Dermatology and Venereology of the Yalgado Ouédraogo University Hospital (CHUYO): Epidemiological, etiological, and therapeutic aspects
Moussa Harouna1, Eric Arnaud Diendere2, Laouali Salissou 3, Muriel Ouédraogo/Ouédraogo4,5, Gilbert Patrice Tapsoba4,5, Amina Zoungrana/Ouédraogo4,5, Apolline Ouédraogo/Sondo4,6, Nina Korsaga/Somé4,5, Abdoul Kadir Ibrahim Mamadou7, Jean Baptiste Andonaba8, Pascal Niamba4,5, Adama Traoré4,5
3, Muriel Ouédraogo/Ouédraogo4,5, Gilbert Patrice Tapsoba4,5, Amina Zoungrana/Ouédraogo4,5, Apolline Ouédraogo/Sondo4,6, Nina Korsaga/Somé4,5, Abdoul Kadir Ibrahim Mamadou7, Jean Baptiste Andonaba8, Pascal Niamba4,5, Adama Traoré4,5
1Department of Dermatology-Venereology, Dosso Regional Hospital, Niger, 2Department of Internal Médicine, Bogodogo University Hospital, Ouagadougou, Burkina Faso, 3Department of Dermatology-Venereology, National Hospital of Niamey, Niger, 4UFR/SDS Joseph KI ZERBO University, Ouagadougou, Burkina Faso, 5Department of Dermatology-Venereology, CHU Yalgado Ouédraogo Ouagadougou, Burkina Faso, 6Department of Infectious Diseases, CHU Yalgado Ouédraogo Ouagadougou, Burkina Faso, 7Department of Internal Médicine, Dosso Regional Hospital, Niger, 8Department of Dermatology-Venereology, CHU Souro Sanou, Bobo Dioulasso, Burkina Faso
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Introduction: Fever is an important problem at the Department of Dermatology and Venereology of CHUYO. The aim of our study was to analyze the epidemiological, etiological, and therapeutic aspects of fever.
Materials and Methods: This was a cross-sectional study with retrospective data collection lasting from January 1, 2017, to December 31, 2018.
Results: Ninety-four patients out of 235 patients collected were febrile, giving a prevalence of 40%. The mean age of the patients was 42.7 ± 4.008 years, with a sex ratio of 1.08. The clinical diagnoses were predominantly bullous dermatoses, including pemphigus and Lyell’s syndrome. Infectious causes were found in 37.3% of the patients, non-infectious causes in 32.9%, and fevers of undetermined etiology in 9.6%. The main germs isolated were Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae. Antibiotic therapy was administered in 86.7% of the cases, mainly aminopenicillins, in 51.1% and third-generation cephalosporin in 22.3%. Thirteen patients (13.8%) died, with mortality being related to advanced age (p = 0.006) and to recognized pathologies of serious prognosis complicated by nosocomial infection (p = 0.046).
Conclusion: The cause of fever in hospitalized dermatology patients should be determined.
Key words: fever; hospitalization; bullous dermatoses; CHU Yalgado Ouédraogo; Burkina Faso
INTRODUCTION
At the Department of Dermatology, fever in hospitalized patients is a major concern for the staff. Although it is suspected and proven to be related to infections, various etiologies may explain the fever [1,2]. It is observed in more than 30% of hospitalized patients [1]. The prevalence of fever varies according to the literature [3–5].
Fever is a common symptom among adult care seekers in sub-Saharan Africa [6], yet it has not been studied epidemiologically by dermatology.
The objective of our study was to analyze the epidemiological, etiological, and therapeutic characteristics of fever in patients hospitalized at the Department of Dermatology and Venereology of Yalgado Ouédraogo CHU in Burkina Faso from January 1, 2017, to December 31, 2018.
MATERIALS AND METHODS
This was a retrospective, cross-sectional study conducted at the Department of Dermatology and Venereology from January 1, 2017, to December 31, 2018. We collected all records of patients hospitalized during this period. This was a simple, exhaustive sample. All patients with fever on admission or during hospitalization were included. The variables studied were socio-demographic characteristics (age, sex, socio-professional status, origin) and clinical and paraclinical data.
We considered the following etiological groups:
- Community infection: any patient admitted with fever with or without an infectious sign of call.
- Nosocomial infection: any patient whose fever occurred more than forty-eight hours after hospitalization with no signs of infection on admission.
- Non-infectious/infectious: a febrile patient admitted with the diagnosis of a non-infectious condition with superinfected lesions and no other infectious sites.
- Indeterminate: any patient with fever on admission and/or hospitalization without a clinical or paraclinical diagnosed focus.
- Non-infectious: patients with fever on admission and/or hospitalization in whom paraclinical investigations did not support an infectious cause.
The data collected was processed and analyzed with SPSS, version 20. The search for a significant difference in the level of knowledge between the different variables was performed with the chi-squared statistical test. The significance level was 5%. The confidentiality of the patients was respected during the exploitation of the files.
RESULTS
Sociodemographic Characteristics
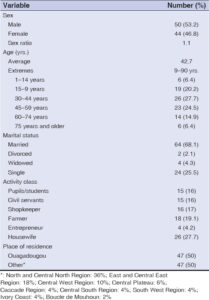
Two hundred and thirty-five patients were hospitalized during the study period. Fever was found in 94 patients, giving a prevalence of fever of 40% (94/235). The mean age was 42.7 ± 4.008 years with extremes of 9 and 90 years. The sex ratio (male-to-female) was 1.13. Housewives and farmers were the most represented, with 27.7% (26/94) and 19.1% (18/94), respectively. The patients resided in the city of Ouagadougou in 50% of the cases; the rest came from regions outside Ouagadougou, with the majority residing in the Sahel region and the middle east (Table 1).
Clinical, Etiological, and Paraclinical Characteristics
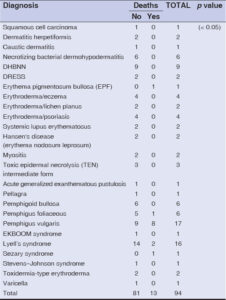
Table 2 and Fig. 1 summarize the clinical and etiologic features.
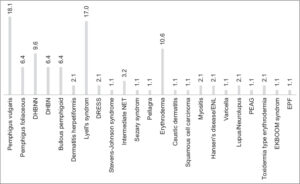
Pemphigus vulgaris (18.1%) and Lyell’s syndrome (17%) were the predominant etiologies for hospitalization (Fig. 2).
Infectious causes predominated in 37.3% of the cases (community-acquired infection in 30.9% and nosocomial infection in 6.4%).
Community infections occurred in patients with no underlying dermatological pathology in 51.6% of the cases.
Nosocomial infections predominated in patients with the diagnoses of pemphigus vulgaris and Lyell’s syndrome in 50% and 33.3%, respectively.
Patients with pemphigus vulgaris predominated with 31.6% (6/19) in non-infectious/infectious etiologies.
Pemphigus vulgaris and Lyell’s syndrome were predominant in the same proportions (22.2%) in the dermatological field of occurrence of fever of undetermined etiology.
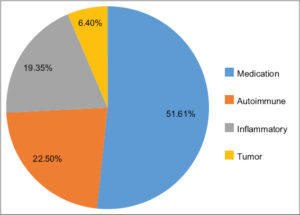
Drug-related causes predominated with 51.6%, followed by autoimmune causes (22.5%) in non-infectious etiologies (Fig. 1).
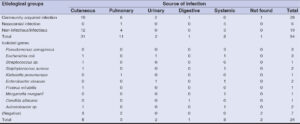
In addition, cutaneous sites were the most frequent (in 31 cases), followed by pulmonary and systemic sites in 11 and 8 cases, respectively.
In the three infectious situations, cutaneous sites were the most frequent (in 31 cases), followed by pulmonary and systemic sites in 11 and 8 cases, respectively (Table 3).
The most frequently isolated pathogenic bacteria were equally Pseudomonas aeruginosa 12.5% (3/24) and Escherichia coli 12.5% (3/24), followed by Staphylococcus aureus 8.3% (2/24) (Table 3).
Therapeutic and Evolutionary Characteristics
A total of 86.2% (81/94) patients had been treated with antibiotics, among which nine had received several families of antibiotics. The most frequently prescribed antibiotic classes were aminopenicillins and third-generation cephalosporin. Amoxicillin + clavulanic acid, ceftriaxone, and metronidazole were the most prescribed antibiotics (Table 4).
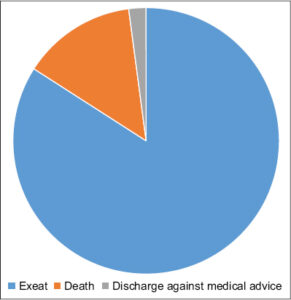
The majority of our patients (79/94; 84%) were cured or improved, yet 13/94 (13.8%) died (Fig. 3).
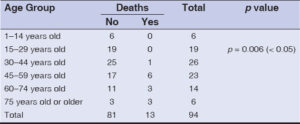
The age of the patients was statistically correlated with the observed deaths (p = 0.006), and the diagnosis of febrile patients had a significant influence on deaths (p = 0.046) (Tables 5 and 6).
DISCUSSION
The main limitations of our study were the fact that some complementary examinations were not performed due to the financial constraints of the patients and the highly limited literature on fever by dermatology, particularly in Africa. The prevalence of fever in our study was 40%. This prevalence varies according to the literature. Göktay reported a fever rate of 16.2% [1]. Gowan in Atlanta and Moon in Korea reported 29% and 5%, respectively [3,4]. As observed by Sandwidi et al. in Ouagadougou, Burkina Faso [7], this may be explained by the context of care in our hospital marked by insufficient hygiene and a high frequency of infections associated with care. We found a predominance of infectious causes of 37.3% (community and nosocomial infections), which is close to 38.6% obtained in a study by Göktay in Turkey [1]. Meanwhile, Gowan in Atlanta, Circiumaru et al. in London, and Goto in Japan reported 53%, 53%, and 54%, respectively [3,8,9] in patients hospitalized at various departments (medicine, surgery, gynecology, pediatrics). This typology of patients could explain the difference compared to our specifically dermatological study population. The high frequency of infectious causes in our study in dermatology could be explained by the loss of skin barrier defense mechanisms related to skin detachments due to underlying dermatoses. Nosocomial infection was accounted in 6.4% and was lower than in a study by Dridi in Tunisia [10] with 13%, and in a study by Gowan with 9% [4] in patients at various departments. Higher rates have been reported in some studies in Africa: Dissou et al. in Benin in 2016 [11], Amona et al. in Congo-Brazzaville in 2016 [12], Zoungrana in Burkina Faso in 2011 [13], and Keita et al. in Conakry in 2016 [14], in 9.8%, 9.41%, 23.7%, and 20%, respectively. While in Germany in 2003, Dettenkofer reported a prevalence of 2.5% at a dermatology department [15]. This may be explained by a notorious lack of hygiene conditions and management of waste from care in our context [7] on the one hand, and the large skin detachments exposed to germs and other invasive procedures found in our patients on the other hand, which constituted factors favoring nosocomial infections [5]. Community infections represented 30.9% of the cases. This observation is comparable to that by Göktay, who reported 24.4% of community infections with a predominance of skin and soft tissue infections, followed by pulmonary infections [1]. This could be explained by the climatic conditions (heat and humidity), which contribute to the alteration of cutaneous defense mechanisms [16]. A significant number of our patients came from the Sahel region and the middle-eastern part of Burkina Faso, which are areas with a hot and humid climate; yet also the frequent use of traditional treatment complicates the skin lesions in our context. Non-infectious causes accounted for 32.9% of febrile patients with diagnoses of autoimmune bullous dermatoses, severe toxidermia, and erythroderma. Göktay observed a predominance of pustular psoriasis, erythroderma, erythema nodosum, and anticonvulsant hypersensitivity syndrome in this group [1]. This could be explained by the inflammatory context and thermoregulatory disturbances secondary to skin integrity damage. The cause of fever was unknown in (9.6%) of our cases. This rate is higher than that reported by Göktay in Turkey (6.3%) [1]. This could be explained by the retrospective nature of our study and the limited technical facilities in our setting. Patients with the diagnosis of pemphigus vulgaris predominated, with 31.6% (6/19), in this group. Inflammation and superinfection seemed to be interrelated and concomitantly responsible for the fever [22]. In our study, cutaneous, pulmonary, and systemic foci predominated. These were mainly bacterial dermohypodermatitis, superinfection of the skin lesions of bullous dermatoses, pneumopathy, and Gram-negative bacteremia. This could be explained by the cutaneous portal of entry, invasive procedures, and the selection of germs from the hospital flora, which are favorable conditions for the creation of serious opportunistic germ infection [16]. We reported a bacteriological profile largely dominated by Gram-negative bacteria with a predominance of Pseudomonas aeruginosa (12.5%), Escherichia coli (12.5%), Staphylococcus aureus 8.3%, Klebsiella pneumoniae 4.1%, Acinetobacter sp. (4.1%), Proteus mirabilis (4.1%), and Morganella morganii (4.1%). This bacteriological profile was reported by some authors in Africa in varying proportions: Dissou et al. in Benin in 2016 [11], Amona et al. in Congo Brazzaville in 2016 [12], and Bassolé in Ouagadougou [17]. This could be explained in our context by the bare skin, which loses all its defense capacities and, thus, becomes an easy entry point for virulent germs to the hospital flora. The previous antibiotic-based treatment in 43.8% and traditional treatment in 41% were linked to the frequent use of self-medication and traditional therapy in our context [18]. This finding was reported by Moon et al. in Korea, who reported that 90.4% of their patients had received traditional herbal treatment and 29.6% had received empirical antibiotic therapy [4]. Moreover, we observed no correlation between the previous use of antibiotics and the appearance of fever in our patients, which should have led us to think of drug-induced fever [19]. The therapeutic management was antibiotic therapy in 86.2% of the cases based on aminopenicillins (amoxicillin + clavulanic acid), third-generation cephalosporin (ceftriaxone), and a combination of several families of antibiotics (aminoglycosides, imipenem). In a study by Göktay et al., 66.7% of patients had received antibiotic therapy [1]. This could be explained by the predominance of infectious causes and the germs isolated by culture in our study. The average length of stay of our patients was 31 days. This compared with 22.2 ± 15.7 days for Sen [20]. The main diagnoses were toxidermia and bullous dermatoses requiring a long hospital stay. We recorded 13.8% of deaths. Chowdhury reported 31.1% [21] and Pires 25% [22]. The diagnosis of severe pemphigus and toxidermia complicated by nosocomial infection was correlated with mortality (p = 0.046) as was advanced age (p = 0.006). This may be explained by the complications of skin loss, including sepsis and Pseudomonas aeruginosa infection, which as described frequently colonizes oozing or acantholytic dermatosis and is accompanied by high morbidity and mortality [23,24] in addition to the severity of these dermatoses.
CONCLUSION
This is the first study in our context. Fever in hospitalized dermatology patients is associated with an infection that complicates the prognosis of bullous dermatoses and Lyell’s syndrome. Invasive procedures were involved in the occurrence of fever in hospitalization. Mortality was (13.8%) attributed to the age and severity of bullous dermatoses complicated by nosocomial infection. Awareness of hygiene and compliance with strict aseptic measures by staff and attendants could reduce the frequency of these infections.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Göktay F, Ceran N, Aydıngöz İE, Mansur AT. Characteristics of fever, etiologic factors, antibiotic use and prognosis in febrile dermatology inpatients:Fever in dermatology inpatients. Int J Dermatol. 2013;52:1331-7.
2. Kaul DR, Flanders SA, Beck JM, Saint S. Brief report:Incidence, etiology, risk factors, and outcome of hospital-acquired fever:A systematic, evidence-based review. J Gen Intern Med. 2006;21:1184-7.
3. Gowan Mc, John E, Richard CR, Norman FJ, Dennis RS, Robert WH. Fever in hospitalized patients with special reference in the medical service. Am J Med. 1987;82:582-5.
4. Moon S, Park K-H, Lee MS, Son JS. Hospital-acquired fever in oriental medical hospitals. BMC Health Serv Res. 2018;18:88.
5. Espinasse F, Page B, Cottard-Boulle B. Risques infectieux associés aux dispositifs médicaux invasifs. Rev Francoph Laborat. 2010;2010:51-63.
6. Carugati M, Kilonzo KG, Crump JA. Fever, bacterial zoonoses, and One Health in sub-Saharan Africa. Clin Med. 2019;19:375-80.
7. Sandwidi L, Traore A, Grau D, Morvan M, Paquis MP, Nana FW, et al. Audit en hygiène hospitalière au CHU YALGADO OUEDRAOGO. 3èColloque GERES en AFRIQUE. Risque infectieux:sécuritédes soignants et qualitédes soins. Casablanca, Maroc 2016:10.
8. Circiumaru B, Baldock G, Cohen J. A prospective study of fever in the intensive care unit. Int Care Med. 1999;25:668-73.
9. Goto M, Koyama H, Takahashi O, Fukui T. A retrospective review of 226 hospitalized patients with fever. Intern Med. 2007;46:17-22.
10. Dridi E, Chetoui A, Zaoui A. [Investigation of the prevalence of nosocomial infection in a Tunisian regional hospital]. Sante Publique. 2006;18:187-94.
11. Dissou A, Anthelme A, Faridath M, Frederic S, Angele KA, Severin A, et al. Facteurs associes aux infections bactériennes nosocomiales dans les services de médecine de l’hôpital universitaire Hubert KOUTOUKOU MAGA de Cotonou, Benin. 3èColloque GERES en AFRIQUE. Risque infectieux:sécuritédes soignants et qualitédes soins. Casablanca, Maroc 2016:8.
12. Amona M, Kokolo B, Loumouamou ML, Mbita AM, Ayah JP, Konongo ALT, Ibata P, et al. Profil bactériologique des infections nosocomiales au service de médecine interne de l’hôpital central des armées de Brazzaville-Congo. 3èColloque GERES en AFRIQUE. Risque infectieux:sécuritédes soignants et qualitédes soins. Casablanca, Maroc 2016:12.
13. Zoungrana KJ, Traore A, Ouédraogo L. Enquête de prévalence des infections nosocomiales au CHUYO de Ouagadougou (BURKINA FASO). ICPIC 2011:16
14. Keita AK, Doumbouya N, Sow MS, KonatéB, Dabo Y, Panzo DA, et al. [Prevalence of nosocomial infections in two hospitals in Conakry (Guinea)]. Sante Publique. 2016;28:251-5.
15. Dettenkoffer M, Wilson C, Ebner W, Norgauer J, Ruden H, Dashner FD. Surveillance of nosocomial infections in dermatology patients in a Germany university hospital. Br J Dermatol. 2003;149:620-3.
16. Boulanger N, Jaulhac B, Lipsker D. Microbiote cutanéhumain normal etmécanismes de défense contre l’infection. Dermatoses microbiennes dans Saurat J-H Lipsker D, Thomas L, Borradori L, Lachapelle J-M. Dermatol Inf Sexu Transmissib. 2017:92-133.
17. BassoléI. Profil bactériologique des suppurations post-opératoires dans les services de chirurgie digestive et chirurgie traumatique du Centre hospitalier universitaire Yalgado Ouédraogo. Thèse. Pharm. n°256 UFR/SDS;Ouagadougou 2012:140p.
18. Nicolas J-P. Plantes médicinales pour le soin de la famille au Burkina Faso. 2009;4. Site:www.jardinsdumonde.org
19. Vodovar D, Le Beller C, Lillo-Le-Louet A, Hanslik T, Megarbane B. [Drug-induced fever:a diagnosis to remember]. Rev Med Interne. 2014;35:1838.
20. Sen A, Chowdhury S, Poddar I, Bandyopadhyay D. Inpatient dermatology characteristics of patients and admissions in a tertiary level hospital in Eastern India. Indian J Dermatol. 2016;61:561-4.
21. Chowdhury S, Podder I, Saha A, Bandyopadhyay D. Inpatient mortality resulting from dermatological disorders at a tertiary care center in Eastern India:A record-based observational study. Indian J Dermatol. 2017;62:518.
22. Pires CAA, Viana VB, Araújo FC, Müller SFR, Oliveira MS de, Carneiro FRO. Evaluation of cases of pemphigus vulgaris and pemphigus foliaceus from a reference service in Parástate, Brazil. An Bras Dermatol. 2014;89:556-61.
23. Morand A, Morand J-J. [Pseudomonas aeruginosa in dermatology]. Ann Dermatol Venereol. 2017;144:666-75.
24. Mamoudou S, Lassina D, Fla K. [Pseudomonas aeruginosa infections in the service of infectious diseases at CHU YO, Burkina Faso:about two cases]. Pan Afr Med J. 2015;21:78.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|






Comments are closed.