Intralesional measles-mumps-rubella vaccine in recurrent common warts: A placebo-controlled study
Shishira R Jartarkar, Manjunath Kadnur, Spoorthy Babu , Swayamsidda Mishra
, Swayamsidda Mishra
Department of Dermatology, Venereology, and Leprosy, Vydehi Institute of Medical Sciences and Research Centre, Bengaluru, India
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Cutaneous warts cause immense an burden to patients as well as physicians. Although most resolve spontaneously within two years, treatment is sought for pain alleviating and cosmetic reasons. Various modalities of treatment are known. The destructive methods are unsuitable for multiple warts and are associated with chances of recurrence, scarring, and pain. In contrast, immunotherapy boosts the host immune response against the virus and helps in clearance, even in distant warts, without scars or physical change. This study was undertaken to assess the efficacy of intralesional MMR vaccine in multiple recurrent common warts.
Materials and Methods: Sixty-six patients with recurrent common warts were divided equally into two groups. In group one, 0.5 mL of the MMR vaccine and, in group two, 0.5 mL of normal saline were injected intralesionally into the base of the largest wart. The sessions were repeated once in two weeks for a maximum of four sessions. The patients were followed up for twelve months to detect recurrences.
Results: Complete clearance of warts was noted in 75.76% (n = 25) of the patients in the study group, whereas, in the control group, 78.79% (n = 26) patients showed no response. The result was statistically significant (p < 0.01).
Conclusion: Intralesional MMR is a safe and effective treatment option for recurrent common warts with minimal side effects.
Key words: viral warts; intralesional MMR vaccine; immunotherapy
INTRODUCTION
Warts are common epidermal proliferations caused by human papilloma virus (HPV) [1]. There are around 180 strains of HPV known to infect epithelial cells with a predilection toward cutaneous and mucosal surfaces [2]. Morphologically, they are classified as verruca vulgaris, plane, filiform, myrmecia, and mosaic warts. Meanwhile, based on their location, they are divided into plantar, palmar, periungual, and anogenital warts [3]. Despite the availability of a wide range of treatment options ranging from medical agents to surgical excision, they pose a therapeutic challenge because of their recurrent nature [4]. These modalities also carry some drawbacks, such as discomfort, scarring, and inefficiency to treat multiple warts [4,5]. However, immunotherapeutic modalities stimulate the host immune system, mainly cell-mediated immunity, which helps in removing the virus without scars or physical change. Various antigens have been attempted both intralesionally and orally, such as vitamin D, interferon, purified protein derivative (PPD), candida antigen, and oral drugs such as levamisole, zinc, and cimetidine [6].
The MMR vaccine is a live attenuated vaccine that provides protection against measles, mumps, and rubella. The intralesional MMR vaccine aids in the clearance of warts via its immunomodulatory action and the stimulation of both humoral and cell-mediated immunity [7].
The aim of this study was to evaluate the safety and efficacy of intralesional MMR injection in multiple recurrent common warts.
MATERIALS AND METHODS
Study Design
This was a hospital-based, single-blind, placebo-controlled, interventional study conducted in the outpatient department of dermatology from June 2018 to January 2020 after obtaining clearance from our institutional ethics committee.
Patients
Sixty-six patients with multiple recurrent common warts were enrolled in the study after obtaining an informed written consent. The patients were divided randomly into two groups with the “chit in the box” method, each containing 33 patients.
Inclusion Criteria
Included were patients with multiple recurrent common warts of different sizes and duration with or without distant warts, willing to provide informed written consent.
Exclusion Criteria
Excluded were the following: children younger than twelve years old, pregnant and lactating females, patients with a keloidal tendency, anogenital warts, patients who had received treatment in the prior four weeks, patients with immunosuppression, a systemic or dermatological disorder, or hypersensitivity to the MMR vaccine.
Method
All patients underwent a protocol of complete history taking and systemic and cutaneous examination.
Complete history taking included the demographic details and present history (disease duration, presence or absence of distant warts, drug intake, systemic illnesses).
The cutaneous examination included the assessment of the common warts, their number, sizes, and the presence or absence of distant warts.
Thorough general and systemic examinations were performed to exclude systemic diseases.
All patients were subjected to HIV testing, with pre- and post-test counseling; those who tested negative were included in the study.
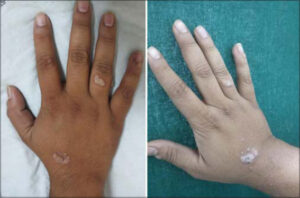
Photographs were taken prior to treatment (baseline) and on each visit.
Group One (Study Group)
Tresivac (freeze-dried MMR vaccine) in single-use vials stored at 2–8°C was employed. It was reconstituted with 0.5 mL of distilled water. Under aseptic precautions, 0.5 mL of MMR vaccine was injected intralesionally into the base of the largest wart with a 30G insulin syringe.
Group Two (Control Group)
Under aseptic precautions, 0.5 mL of normal saline was injected into the base of the largest wart.
Procedure
After cleaning the lesions with povidone-iodine and spirit, injections were given only into the base of the largest wart with a 40-U insulin syringe. The syringe was held parallel to the skin surface and the needle was held with the bevel facing upwards while injecting in all patients. In both groups, the injections were repeated every two weeks until complete clearance or for a maximum of four sessions.
The patients were followed up for twelve months after the last dose to detect recurrences. The patients were evaluated for clearance of warts and adverse effects.
Post-Treatment Care
No other treatments for cutaneous warts were employed concurrently or during the follow-up period.
Assessment of Improvement
The clinical improvement was assessed with color photographs taken at baseline, on each session, and twelve months after the last session.
The clinical improvement was graded as:
-
• complete clearance: the disappearance of warts with normal-looking skin;
-
• partial response: a reduction in size and/or number of the warts;
-
• no response: no change in size or number.
Statistical Analysis
The statistical analysis was performed with the SPSS software, version 22. Frequencies and percentages were employed for categorical variables, whereas means were employed for quantitative variables. The Chi-squared test and t-test were performed. A p value below 0.05 was considered significant.
RESULTS
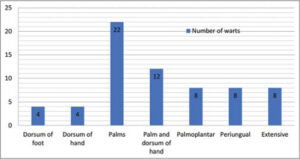
All 66 patients completed the study. The patients were comparable with respect to age and sex (p > 0.05). The number and location of warts observed in our study were noted (Table 1 and Graph 1). The mean age in the study group (group one) was 29.1 ± 6.46 and, in the control group (group two), it was 28.7 ± 6.72. In our study, we noticed a male predominance in both groups: in the study group, 18 patients (54.55%) and, in the control group, 17 patients (51.52%) (Table 2).
 |
Table 1: Numbers and sites of the warts. |
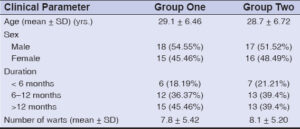 |
Table 2: Demographic data of the participants. |
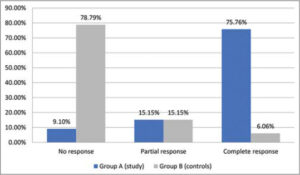
In the study group, complete clearance was noted in 75.76% (n = 25) (Fig. 1), and partial clearance was noted in 15.5% (n = 5) of the patients. No improvement was noted in 9.1% (n = 3) of the patients (Fig. 2). The mean number of injections required for complete clearance of warts was 3.24.
In the control group, 78.79% (n = 26) of the patients showed no improvement and 15.15% (n = 5) showed partial improvement. Only 6.06% (n = 2) of the patients showed complete response (Graph 2). The difference in improvement was statistically highly significant (p < 0.01. The complete resolution of distant warts was noted in all patients who had a complete response (Table 3).
| Table 3: Clinical improvement in the cases and controls. |
Side Effects
Pain during injection was noted in 81.82% (n = 27) of the patients in the study group and in 21.21% (n = 7) in the controls. Flu-like symptoms were noted in 12.12% (n = 4) of the patients, which subsided spontaneously within 48–72 hours. The patients were followed up for twelve months and there was no recurrence in patients with the complete resolution of warts.
DISCUSSION
Recurrent multiple warts bring social stigma to the patient as well as a challenge to the dermatologist. No single therapy had proven to be completely effective, especially if numerous and distant lesions are considered.
Immunotherapy is a type of biological therapy that employs substances to modify the immune response to help the body to fight an infection, cancer, or autoimmune disease [8].
It is a preferred treatment option due to its effect on both treated and distant warts by inducing HPV-targeted immunity. Intralesional MMR stimulates the adaptive immune system and activates natural killer (NK) cells. It induces a type 1 helper T-cell mediated delayed-hypersensitivity reaction against the antigen and the HPV-infected cells. This causes the destruction of the virus and infected host cells, not only in the treated warts yet also the distant warts [9,10]. The MMR vaccine is more immunogenic than other skin test antigens. Due to the presence of three synergistic viral antigens, a stronger immune response against HPV is generated via the production of cytokines such as interleukin (IL)-2,4,5 and TNF-a [11,12].
Our study revealed that intralesional MMR vaccine is an effective therapy for multiple recurrent common warts. Twenty-five out of the thirty-three patients (75.76%) showed the complete clearance of warts. Comparable results were reported by Chauhan et al., with 82.4% (42/51) of patients showing complete clearance [13]. Similar results were also observed by Nofal et al., with 81.4% of patients achieving complete healing [14]. Intralesional MMR vaccine was found to be safer and more effective than cryotherapy in a study conducted by Abd El-Magiud et al. [15].
During the follow-up period of twelve months, no recurrence was noted in our study group, which was in concordance with Nofal et al., who reported no recurrence in the MMR group after six months [14].
A placebo-controlled study by Awal and Kaur using 0.5 mL of intralesional MMR vaccine every two weeks for a maximum of five sessions showed complete clearance in 68% of patients [12].
A prospective, randomized study by Saini et al. comparing the efficacy of 0.3 mL intralesional MMR vaccine and 100% topical TCA every two weeks for a total of three sessions noted a more than 75% improvement in 49.43% of patients, with 26.44% showing complete response in the MMR group. Meanwhile, 11.11% had a more than 75% improvement, with only 7.94% showing complete resolution in the TCA group [9].
Pain during injection and flu-like symptoms were the side effects reported by our patients. This was comparable to various other studies [9,12,13]. The limitation of our study was a small sample size.
CONCLUSION
Intralesional MMR vaccine for common warts appears to be a simple, safe, and promising treatment modality with a low recurrence rate. The eradication of distant warts with no scarring and pigmentation are its added benefits. However, further, large, well-designed case–control studies are required to delineate the dosing regimen and duration for its effective use.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Stanley MA. Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev. 2012;25:215-22.
2. Groves IJ, Coleman N. Pathogenesis of human papillomavirus-associated mucosal disease. J Pathol. 2015;235:527-38.
3. Salman S, Ahmed MS, Ibrahim AM, Mattar OM, El-Shirbiny H, Sarsik S, Afifi AM, Anis RM, Yakoub Agha NA, Abushouk AI. Intralesional immunotherapy for the treatment of warts:A network meta-analysis. J Am Acad Dermatol. 2019;80:922-30.e4.
4. Bacelieri R, Johnson SM. Cutaneous warts:An evidence-based approach to therapy. Am Fam Physician. 2005;72:647-52.
5. Rivera A, Tyring SK. Therapy of cutaneous human papillomavirus infections. Dermatol Ther. 2004;17:441-8.
6. Vender R, Bourcier M, Bhatia N, Lynde C. Therapeutic options for external genital warts. J Cutan Med Surg. 2013;17 Suppl 2:S61-7.
7. Zamanian A, Mobasher P, Jazi GA. Efficacy of intralesional injection of mumps-measles-rubella vaccine in patients with wart. Adv Biomed Res. 2014;3:107.
8. Thappa DM, Chiramel MJ. Evolving role of immunotherapy in the treatment of refractory warts. Indian Dermatol Online J. 2016;7:364-70.
9. Saini S, Dogra N, Dogra D. A prospective randomized open label comparative study of efficacy and safety of intralesional measles, mumps, rubella vaccine versus 100% trichloroacetic acid application in the treatment of common warts. Int J Res Med Sci. 2016;4:1529-33.
10. Johnson SM, Horn TD. Intralesional immunotherapy for warts using a combination of skin test antigens:A safe and effective therapy. J Drugs Dermatol. 2004;3:263-5.
11. Na CH, Choi H, Song SH, Kim MS, Shin BS. Two-year experience of using the measles, mumps and rubella vaccine as intralesional immunotherapy for warts. Clin Exp Dermatol. 2014;39:583-9.
12. Awal G, Kaur S. Therapeutic outcome of intralesional immunotherapy in cutaneous warts using the mumps, measles, and rubella vaccine:A randomized, placebo-controlled trial. J Clin Aesthet Dermatol. 2018;11:15-20.
13. Chauhan PS, Mahajan VK, Mehta KS, Rawat R, Sharma V. The efficacy and safety of intralesional immunotherapy with measles, mumps, rubella virus vaccine for the treatment of common warts in adults. Indian Dermatol Online J. 2019;10:19-26.
14. Nofal A, Nofal E. Intralesional immunotherapy of common warts:Successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol. 2010;24:1166-70.
15. Abd El-Magiud EM, Abd El-Samea GM, Gaber HD. Intralesional injection of measles, mumps, and rubella vaccine versus cryotherapy in treatment of warts:A randomized controlled trial. Dermatologic Therapy. 2020;33:e13257.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-2283-8050 http://orcid.org/0000-0003-2283-8050 |





Comments are closed.