Multiple autoimmune syndrome (vitiligo with Crohn’s disease and thyroid disease) in a single patient: A variant type
1Department of Dermatology, Tikrit University, College of Medicine, Iraq, 2Internal Medicine Department, Tikrit University, College of Medicine, Iraq
Citation tools:
Copyright information
© Our Dermatology Online 2023. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
The co-existence of different autoimmune disorders in the same individual is known as multiple autoimmune syndrome (MAS), which was described by Humbert and Dupond in 1988. Pathogenesis in the immune system occurs with more recurrence in patients with a background marked by another immune disorder. Around 25% of patients with immune system sicknesses tend to foster more immune disorders. In MAS, patients frequently have, at any rate, one dermatological condition, usually vitiligo or alopecia areata. Herein, we report a case of MAS in a sixteen-year-old male suffering from vitiligo with Crohn’s disease and thyroid disease.
Key words: Multiple Autoimmune Syndrome (Mas); Inflammatory Bowel Disease (Ibd); Gastrointestinal Tract (Git); Crohn’s Disease (Cd)
INTRODUCTION
Vitiligo is an acquired pigmentary anomaly of the skin manifested by white, depigmented patches surrounded by a normal or hyperpigmented border [1]. There is a complete loss of melanocytes in the affected areas [2]. Most patients with vitiligo have no other associated findings; however, vitiligo has been reported to be associated with alopecia areata, hypothyroidism, Graves’ disease, Addison’s disease, pernicious anemia, insulin-dependent diabetes mellitus, uveitis, chronic mucocutaneous candidiasis, polyglandular autoimmune syndromes, and melanoma [3].
Inflammatory bowel disease (IBD) is a chronic, immune-mediated disorder comprised of Crohn’s disease and ulcerative colitis [4]. The etiology of IBD remains unclear; however, recent research indicates that the pathophysiology of IBD involves abnormalities in disease susceptibility genes, environmental factors, and intestinal bacteria [5]. Crohn’s disease (CD) is a compulsive idiopathic IBD that chiefly arises in the small intestine and at the beginning of the large intestine. CD results from T-cell initiated characteristic inflammation caused usually by innocuous commensal bacteria or bacterial products. In CD, the covering of the gastrointestinal tract becomes inflamed. Any portion of the tract may be altered, although generally the ileum and colon are affected [6].
Thyroid sickness happens with either unusually raised or lowered thyroid hormones. Hyperthyroidism is, for the most part, described by an overabundance of thyroid hormones with diminished serum thyroid-stimulating hormone and raised triiodothyronine and thyroxine concentrations. Conversely, hypothyroidism is, by and large, portrayed by diminished thyroid chemical blend and raised thyroid-stimulating hormone, as well as low triiodothyronine and thyroxine concentrations [7]. The most common cause of thyroid dysfunction is an iodine deficiency, and two billion individuals are estimated to have insufficient iodine intake. In countries with routine iodine supplementation, however, autoimmune thyroid disorders are the most common causes of thyroid disorders [8].
MAS is defined as the occurrence of at least three autoimmune diseases in the same patient [9]. The most frequent autoimmune diseases in such patients include dermatological conditions such as alopecia areata and vitiligo [10].
The pathogenesis of MAS is unknown. However, the autoimmune tautology theory proposes that autoimmune diseases share common immunogenic, physiopathological, and genetic mechanisms. This may lead to the presentation of similar signs and symptoms, demonstrating their common origin [11].
MAS may be divided into three gatherings, as indicated by the commonness of their relationship. Class one comprises myasthenia gravis, thymoma, polymyositis, and giant cell myocarditis. Class two comprises Sjögren’s syndrome, rheumatoid arthritis, primary biliary cirrhosis, scleroderma, and autoimmune thyroid disease. Class three comprises autoimmune thyroid disease, myasthenia and/or thymoma, Sjögren’s syndrome, pernicious anemia, idiopathic thrombocytopenic purpura, Addison’s disease, insulin-dependent diabetes, vitiligo, autoimmune hemolytic anemia, systemic lupus erythematosus, and dermatitis herpetiformis. For this group, HLA-B8 and/or -DR3 or -DR5 seem to be an important factor [12].
CASE REPORT
The sixteen-year-old Iraqi male who presented to the clinic was first diagnosed with vitiligo when he was nine years old. His family history included vitiligo in her mother. A dermatological examination revealed multiple, well-demarcated, depigmented macules and patches mostly on the legs and arms (Figs. 1 and 2) and scattered lesions on the trunk and face; the hair, anogenital area, nails, and oral cavity were normal. Wood’s lamp examination was employed to diagnose the hypopigmented patches on the trunk as vitiligo. Non-segmental vitiligo of the generalized type was diagnosed, for which he was treated by others doctors with a topical steroid preparation and topical methoxsalen. There was a moderate response to the treatment with some repigmentation.
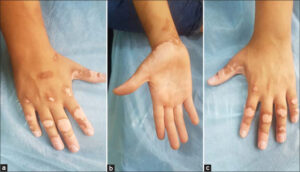 |
Figure 1: (a-c) Multiple depigmented patches on both hands. |
 |
Figure 2: (a-c) Multiple depigmented patches on the elbows and knees. |
His past medical history revealed that he had had gastrointestinal symptoms such as abdominal pain, diarrhea, and weight loss since 2012. He underwent colonoscopy and histopathology in July 2020. Colonoscopy revealed a small, painful, low-rectal ulcer, and a biopsy was taken from the ileal, colonic, and rectal sites. A diagnosis of Crohn’s disease was established by colonoscopy and histopathological examination.
Laboratory studies disclosed the following values: iron-deficiency anemia (serum ferritin: 8.83; hematocrit: 36.8%; hemoglobin: 12 g/dL), inflammatory syndrome (ESR: 16 mm/1 h; CRP > 5 mg/dL), renal test (urea: 16.71 mg/dL, creatinine: 0.53, BUN: 8 mg/dL), and liver test (alkaline phosphatase: 288 IU/L).
As for celiac disease testing, antigliadin antibodies were within normal limits, and tissue transglutaminase IgG and IgA were positive.
A thyroid function test revealed the following: T3 (1.3 nmol/L), T4 (72 nmol/L), TSH (15.7 mIU/L). Antithyroid antibody: Anti-Tg antibody (96.14 IU/mL), TPO antibody (934.31 IU/mL), thyroglobulin (0.22 ng/mL).
Antinuclear antibody (ANA) profile and HIV and HCV antibody tests were negative.
The diagnoses were finally established: multiple autoimmune syndrome (vitiligo with Crohn’s disease and thyroid disease) existing in a single patient, a variant type of multiple autoimmune syndrome.
DISCUSSION
Presumably, the link between various autoimmune diseases might be genetic and/or environmental exposures that trigger an aberrant immune response. Although various autoimmune diseases differ in their target organs and antigens, they share a common loss of self-tolerance [13].
The term autoimmune tautology is employed to describe the common physiopathological mechanisms and genetic factors shared by numerous autoimmune diseases, and clinically this is evident in the cases of polyautoimmunity and familial autoimmunity [14]. Polyautoimmunity is likewise significant for the flow conversation since it might impact the seriousness of immune system illnesses. As a matter of fact, some creators contend that there is a more extreme show of a specific promotion when polyautoimmunity is available, while others have tracked down no impact or even a superior forecast in such cases [15].
The case described here was a typical form of MAS by three autoimmune disorders, including vitiligo, hypothyroidism, and Crohn’s disease.
Pathogenesis in the immune system occurs more frequently in patients with a history of other immune system illnesses. The tendency to develop more diseases occurs in around 25% of these patients [16].
In a recent review of the literature, Manuel et al. revealed that MAS type I affected one case in two to three million newborns. Its prevalence in the general population is estimated to be 1/90,000 in Norway and 1/130,000 in Ireland. MAS is thought to be higher in Sardinians and Iranian Jews, with a prevalence of 1/14,000 and 1/9000, respectively. The female sex seems to be a risk factor for polyautoimmunity [17].
In a retrospective analysis of eleven patients with type 3 MAS, Klisnick et al. found that 63.6% of the patients had segmental or bilateral vitiligo, and 90% presented with autoimmune thyroid disease [18].
Cutaneous diseases related to Crohn’s disease are erythema nodosum, pyoderma gangrenosum, epidermolysis bullosa acquisita, polyarteritis nodosa, and vitiligo. The relationship between Crohn’s disease and vitiligo has been observed in the literature. In research by Tanusin et al., the co-existence of vitiligo and Crohn’s disease was seen in 10% of patients. McPoland and Moss revealed an instance of Crohn’s disease and vitiligo. In the two examinations, vitiligo was correspondingly related [19]. Immune system peculiarities may be unmistakable in fiery gut sickness. Ulcerative colitis, specifically, shows a high rate of related immune system sicknesses, including hypothyroidism, essential sclerosing cholangitis, vitiligo, and alopecia areata [20].
CONCLUSION
The finding of MAS relies upon the doctor’s exactness and the time that the principal immune system disease began. The presence of one immune system sickness ought to make one aware to watch for another. Therefore, early diagnosis and proper management are of great importance. As a rule, the presence of one issue in the immune system helps to lead to the revelation of other immunological conditions.
This case report illustrates the distinctive presentation of a case of the clinical coexistence of multiple autoimmune diseases (vitiligo with Crohn’s disease and thyroid disease). The event of numerous immune system peculiarities in this situation shows the need for reconnaissance for the improvement of emerging immune system sicknesses in the inclined patients.
CONSENT
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. James DW, Elston MD, Treat RJ, Rosenbach AM, Neuhaus MI. Andrew’s diseases of the skin clinical dermatology. Thirteen Edition. Elsevier;2019:871.
2. Weller BR, Hunter J.A Hamish, Mann W.M. Clinical dermatology. FIFTH EDITION. Blackwell Publishing;2014:271.
3. Habif PT. Clinical dermatology:A color guide to diagnosis and therapy. Seven Edition. Elsevier Inc. 2021:774.
4. Malik FT, Aurelio MD. Extraintestinal manifestations of inflammatory bowel disease. Bioline International. March 9, 2022.:1989-2004.
5. Nakase H, Uchino M, Shinzaki S, Matsuura M, Matsuoka K, Kobayashi T. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2021;56:489-526.
6. Sindhu RK, Goyal A, Das J, Neha, Arora S. Crohn’s disease:Symptoms, diagnosis, management by medical and alternative treatment. Pharm Sci Asia. 2021;48:204-23.
7. Malik S, Cohen RP. Vitiligo-Associated autoimmune disorders:A woman with vitiligo and incipient hypothyroidism. Cureus. 2021;13:e19164.
8. Alzahrani SA, Mourad AM, Hafez K, Almaghamsy MA, Alamri AF, Juhani RN. Diagnosis and management of hypothyroidism in Gulf Cooperation Council (GCC) Countries. Adv Ther. 2020;37:3097-111.
9. Alwasaidi AT, Mustafa W, Osman H, Hebshi AA, Sr AA. Multiple autoimmune syndrome with alopecia universalis and immune thrombocytopenic purpura. Cureus. 2021;13:e13033.
10. Mahdi M Sereshki A, Almasi S, Behnam B, Semnani F. Autoimmune haemolytic anaemia and multiple autoimmune syndrome. Eur J Case Rep Intern Med. 2019;6:001111.
11. González CC, Martínez AS, Guanes RJ. Ocular cicatricial pemphigoid, Sjögren’s syndrome, and Hashimoto’s thyroiditis as a multiple autoimmune syndrome:A case report. Eur J Ophthalmol. 2022;32:NP52-5.
12. Madan PM., Ramesh TC. Multiple autoimmune syndrome. Indian J Dermatol Venereol Leprol. 2003;69:298-9.
13. Greenberg MB, Casper CT, Jayne M N, Plumb P, Liang S, Goyal M. Familial history of autoimmune disorders among patients with pediatric multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1049.
14. Prabhu SS, Ravi D, Shenoi DS, Pai K, Sudhir UK. Multiple autoimmune syndrome with isotopic phenomenon:Association of lichen planus, vitiligo and alopecia areata with autoimmune hepatitis. J Pak Assoc Dermatol. 2018;28:356-9.
15. Villarraga RA, Amaya AJ, Rodriguez RA, Mantilla DR, Anaya MJ. Introducing polyautoimmunity:Secondary autoimmune diseases no longer exist. Autoimmune Dis. 2012;2012:254319.
16. Setiyohadi BS, Mokoagow MI. Multiple autoimmune syndrome (Graves’disease, systemic lupus erythematosus, and systemic sclerosis) in a young woman in Jakarta. J Rheumatol.2020;3:41-4.
17. Niasse M, Kane SB, Dimitri A Mabom W, Makougang C, DézoumbéM. Multiple autoimmune syndrome:A study of 25 Senegalese cases. Open J Rheumatol Autoimmune Dis. 2020;10:14-23.
18. Dourmishev L, Pozharashka J, Miteva L. Probable multiple autoimmune syndrome in a patient with vitiligo, autoimmune thyroiditis, and diabetes mellitus:A case report. J Skin Stem Cell. 2019;6:e103596.
19. Gargi R, Hita HM. Mehta, Jhamwar MM. Anogenital Crohn’s disease with vitiligo. Indian J Sex Transm Dis AIDS. 2014;35;53-5.
20. Cojocaru M, Cojocaru MI, Silosi I. Multiple autoimmune syndrome. Mædica (Bucur). 2010;5;132-4.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-1007-0451 http://orcid.org/0000-0003-1007-0451 |





Comments are closed.