Leukocytoclastic vasculitis induced by medications displaying colocalizing lesional deposits for CD15, myeloperoxidase and HLA-DPDQDR: A Yin and Yang?
Ana Maria Abreu Velez 1, Bruce R Smoller2, Michael S. Howard3
1, Bruce R Smoller2, Michael S. Howard3
1Georgia Dermatopathology Associates, Atlanta, Georgia, USA, 2Department of Pathology and Laboratory Medicine, University of Rochester School of Medicine and Dentistry, Rochester, New York, USA, 3Department of Dermatology, University of Rochester School of Medicine and Dentistry, Rochester, New York, USA
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Leukocytoclastic vasculitis is an inflammatory disease of small blood vessels; circulating immune complexes are part of the disease. A 57-year-old female presented with a sudden appearance of palpable purpura with petechial hemorrhages on the lower limbs after taking multiple medications. Skin biopsies were stained for H&E, direct immunofluorescence (DIF) and immunohistochemistry (IHC). The DIF revealed strong staining with multiple immunoglobulins and other markers in small dermal blood vessels, including those around skin appendices The IHC was positive for myeloperoxidase. CD15, myeloperoxidase and HLA-DPDQDR on the upper dermal blood vessels, as well as on inflammatory cells and debris around the vessels. These findings have not been previously documented and may indicate that circulating immune complexes activate the neutrophils.
Key words: Leukocytoclastic vasculitis; CD15; myeloperoxidase; HLA-DPDQDR; direct immunofluorescence; immunohistochemistry
Abbreviations: Hematoxylin and eosin (H&E); immunohistochemistry (IHC); direct immunofluorescence (DIF); basement membrane zone (BMZ); 4′,6-diamidino-2-phenylindole (DAPI)
INTRODUCTION
Leukocytoclastic vasculitis is an inflammatory condition that affects the small blood vessels of the skin but can also affect other organs. In half of the cases leukocytoclastic vasculitis is idiopathic, and infections including viruses and drugs are the most common triggers for secondary leukocytoclastic vasculitis [1,2]. The disease equally affects both sexes. The lesions usually are accompanied by a burning rash predominantly in the lower extremities often associated with pain; other areas of the skin can be affected [1,2]. The most common cutaneous manifestation is a palpable purpura; other lesions include livedo reticularis, bullae, small papules, ulcers, and livedo reticularis. When the lower extremities are affected, is important to search for arthralgias or arthritis involving the knees or ankles [1,2]. Systemic symptoms can occur including fever, arthralgias, fatigue, and malaise in 50 to 60% of patients; thus, is important to perform laboratory studies to rule out other causes.
Histologically leukocytoclastic vasculitis is characterized by deposition of neutrophils, fibrinoid necrosis and neutrophilic debris („leukocytoclasia”) in small dermal postcapillary venules [1,2].
CASE REPORT
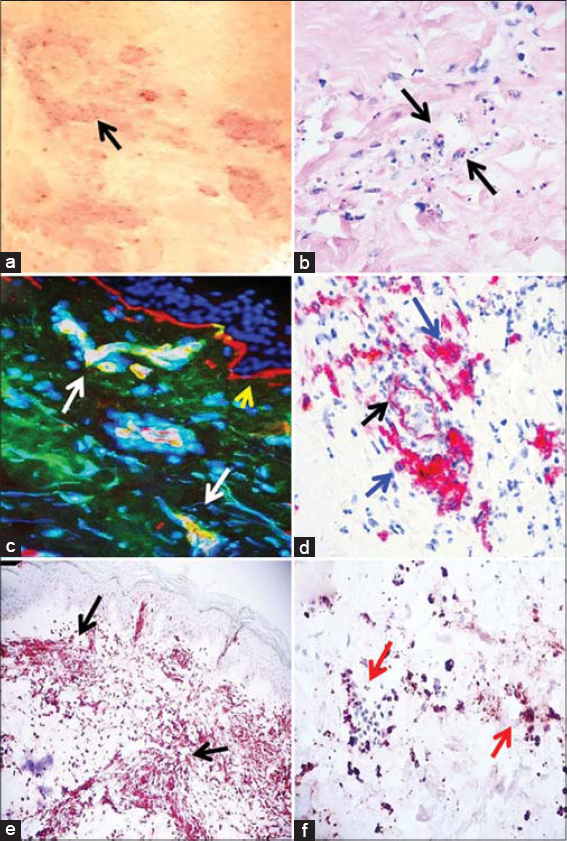
We describe a 57-year-old female who presented with a sudden appearance of hemorrhagic petechiae and plaques, comprising a clinical violaceous and erythematous palpable purpura (Fig. 1). No arthritis or arthralgias were detected. The patient described itchiness and burning sensations on the lesions. The patient was taking clonidine HCL 0.2 mg/day, atenolol 100 mg/day, tramadol HCL 50 mg/day, cyclobenzaprine 10 mg/day, and furosemide 10 mg/day for a constellation of clinical high blood pressure, depression and localized aches and pains. The patient did not exhibit general symptoms, and the possibility of ulcerative colitis was excluded. Laboratory tests including a complete blood count, erythrocyte sedimentation rate, biochemistry profile with liver and renal functions, urinalysis and C-reactive protein were negative. A skin biopsy for hematoxylin and H&E and PAS staining, as well as direct immunofluorescence (DIF) immunohistochemical (IHC) staining were performed. These procedures were performed as previously described [3]. The H&E tissue sections demonstrated an early subepidermal blister with eosinophils and occasional lymphocytes present within the blister lumen. Neutrophils were rare, but leukocytoclastic debris was noted within the lumen. Within the dermis, a moderately florid, superficial, perivascular infiltrate of lymphocytes, histiocytes, neutrophils, neutrophilic debris and rare eosinophils was identified (Fig. 1a). No frank vasculitis was seen. The DIF revealed deposits of FITC conjugated anti-human IgG, anti-kappa, anti-lambda, anti-human complement C3 and C4, anti-human fibrinogen and anti-human albumin (all these positive +++; the scale used was + weak positive, through ++++ strong positive) in 1) most of the upper dermal vessels, 2) some of the vessels communicating with the deep dermal vessel system, 3) mesenchymal/endothelial cell junctions, 4) small vessels around the piloerector muscle basement membrane area and 5) small vessels around eccrine sweat glands. The PAS displayed strong positivity around all the small dermal vessels, including those feeding the skin appendices.
In our workup, we employed both single- and double-color IHC staining, performed with the Leica Bond MAX automated system (Buffalo Grove, Illinois, USA) using a Novolink™ detection and Compact Polymer™ technology as previously described [2]. For primary staining, we used the Bond Max refined red detection DS9390, an alkaline phosphatase linker polymer, and fast red chromogen (red staining). For secondary staining, we used the Bond Max refined brown detection DS9800, a horseradish peroxidase linker polymer, and DAB chromogen (brown staining). Positive and negative controls were consistently performed. The following antibodies were used from Novocastra-Leica for IHC: HLA-DPDQDR antigen, clone CR3/43, polyclonal rabbit anti-human antibodies: myeloperoxidase, and BOND™ Ready-to-Use Primary Antibody CD15 (MMA) Catalog No: PA0473 (Leica Biosystems Newcastle, Newcastle Upon Tyne, United Kingdom). We also utilized CD8 and CD68; cells both were positive in few cells around the vessels. Figure 1c shows positive staining of the vessels using FITC conjugated anti-human fibrinogen. Figure 1d shows an IHC stain, showing positive expression of HLA-DPDQDR on the upper dermal vessels as well as on the inflammatory cells surrounding the vessels. Figures 1e and 1f document simultaneous positivity on IHC staining for myeloperoxidase and CD15.
The clinical and microscopic findings established a diagnosis of leukocytoclastic vasculitis. The patient was re-evaluated by her treating physician to decrease her medication dosages, and to remove the Tramadol. The patient was ordered to rest, elevate her legs and apply ice packs to the affected areas. Loratadine 10 mg/day and Celebrex® (Celecoxib) 200 mg were provided with topical steroids. After treatment, the patient’s skin lesions improved. Repeat DIF and IHCs were performed after 15 days of treatment and were both within normal limits.
DISCUSSION
Leukocytoclastic vasculitis has been associated with numerous etiologic factors including drugs, autoimmune diseases, collagen vascular diseases, infections, foods and their preservatives, hair dyes and malignancies among other causes [1,2]. Although the exact pathogenic mechanism remains to be elucidated, circulating immune complexes are believed to be involved [1,2]. Neutrophils are innate immune cells that generate significant cell debris in cases of leukocytoclastic vasculitis.
In our case, both immunoglobulins and complement were seen in lesional small dermal blood vessels, including those supplying skin appendices. The simultaneous positivity on lesional skin biopsies from a leukocytoclastic vasculitis patient with simultaneous deposition of CD15, myeloperoxidase and HLA-DPDQDR represents a new and interesting finding. We reviewed multiple databases including all the years in the PubMed database and could not find previous documentation. The findings were also positive on vessels supplying skin appendices.
We do not know why the IHC positivity for CD15, myeloperoxidase and HLA-DPDQDR occurred simultaneously. It is accepted that HLA-DPDQDR is classically related to a non-innate, more specialized immune response with lymphocytes. We noted only a few CD8 and CD68 positive cells around the vessels. We speculate that HLA-DPDQDR expression may play an unknown role in exposing neutrophilic and/or endothelial cell molecules that were previously not exposed to the immune system. Thus, this finding warrants more investigation.
Antigen presentation by major histocompatibility complex (MHC) proteins is essential for adaptive immunity. The MHC Class II-encoded HLA-DPDQDR antigens play a crucial role in the human immune response. Their constitutive expression has been classically restricted to several immunocompetent cells defined as antigen-presenting cells. In our case, the expression of the gene at the lesional protein level may suggest a generic predisposition towards a vasculitis in this patient [4].
CD15 antigen (also known as Lewis x, or Lex) is a characteristic marker for human myeloid cells and mediates neutrophil adhesion to dendritic cells [5, 6]. CD15 protein can be found in the cell membranes and the cytoplasm’s of granulocytes and epithelial cells in a variety of tissues. Acute funisitis, a granulocyte-related inflammation of the umbilical cord, is associated with chorioamnionitis and perinatal adverse events. Thus, CD15 immunohistochemistry has been a powerful tool for studying the patterns of clinically relevant umbilical vasculitis, especially in cases that were indeterminate according to morphology alone [5]. To account for our observed presence of CD15, we found a published study that showed that antibody blockade of Lewis X (Lex) blocked chemotaxis [5, 6]. It is known that glycans and endothelial glycan-binding proteins are critical for initial transepithelial migration of neutrophils out of the vasculature. We suggest that the body and the immune system could be trying to counterbalance, and limit neutrophilic flow out of the vasculature to limit secondary vessel damage. Often, the body and the immune system work with balancing mechanisms to avoid further damage.
It was recently demonstrated that although intracellular neutrophil elastase functions as a host defense factor against pathogens, its leakage into spaces induces degradation of host connective tissue components. The authors used a model of pneumococcal pneumonia and were able to demonstrate in cell cultures that expression of HLA class II molecules was decreased in THP-1-derived macrophages treated with supernatants from dead neutrophils [6]. Based on this data and given our findings, we suggest that the neutrophil and CD15 response may be one of „protection” against a specific antigenic response present in this kind of vasculitis; and thus could help the immune system to avoid a further „chronic” immune response via B and T lymphocytes [7].
Neutrophils are known to be effector cells of innate immune responses. However, when stimulated by interferon-γ (IFN-γ) to express HLA-DR, neutrophils acquire accessory cell functions for superantigen-mediated T cell activation [8]. The data was demonstrated by inducing in vitro HLA-DR expression on neutrophils. The findings in our case may represent a parallel example of what could occur in vivo.
We conclude in our case that the demonstration of HLA-DP, DQ, and DR antigens on blood vessels, as well as in the inflammatory infiltrate and cellular debris in the vessel walls may support the concept that the vessels (through their interactions with certain molecules) may play an active role in leukocytoclastic vasculitis. A larger series studying the presence of CD15, myeloperoxidase and HLA DP, DQ, and DR antigens is needed, and could lead to better diagnostic and/or therapeutic tools for this disorder. We also noted involvement of mesenchymal/endothelial cell junctions, small vessels around the piloerector muscle basement membranes and small vessels around the eccrine glands. We suggest that the constellation of these features may represent a non-traditional, expanded leukocytoclastic vasculitic process.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki. The authors certify that they have obtained all appropriate patient consent forms, in which the patient gave their consent for images and other clinical information to be included in the publication. The patient understood that their names and initials will not be published, and due effort was made to conceal their identity.
REFERENCES
1. Pantic I, Jevtic D, Nordstrom CW, Madrid C, Milovanovic T, Dumic I. Clinical manifestations of leukocytoclastic vasculitis, treatment, and outcome in patients with ulcerative colitis:a systematic review of the literature. J Clin Med. 2022;11:739.
2. Baigrie D;Goyal A;Crane JS. Leukocytoclastic Vasculitis StatPearls [Internet]. 2022 Jan-.Last Update:August 11, 2021.
3. Abreu-Velez AM, Smoller BR, Howard MS. Bullous lupus erythematosus with basement membrane deposits of IgD. Our Dermatol Online. 2022;13:1-4.
4. Tumer G, Simpson B, Roberts TK. Genetics, Human Major Histocompatibility complex (MHC). 2021 aug 11. in:Statpearls [internet]. Treasure Island (fl):Statpearls publishing;2022 jan-
5. Brazil JC, Sumagin R, Richard D. Cummings, RD, Nancy A. Louis NA, et al. Targeting of neutrophil Lewis X blocks transepithelial migration and increases phagocytosis and degranulation. Am J Pathol. 2016;186:297-11.
6. Hatano Y, Tamada M, Shiga T, Niwa A, Kanayama T, Tomita H, et al. Clinically relevant umbilical cord inflammation identified based on CD15-associated vasculitis patterning. Placenta. 2021;108:39-46.
7. Domon H, Maekawa T, Isono T, Furuta K, Kaito C, Terao Y. Proteolytic cleavage of HLA class II by human neutrophil elastase in pneumococcal pneumonia. Sci Rep. 2021;11:2432.
8. Reinisch W, Lichtenberger C, Steger G, Tillinger W, Scheiner O, Gangl A. Donor dependent, interferon-γ induced HLA-DR expression on human neutrophils in vivo. Clin Exp Immunol. 2003;133:476-84.
Notes
Source of Support: Georgia Dermatopathology Associates, Atlanta, Georgia, USA.
Conflict of Interest: The authors have no conflict of interest to declare.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-7692-4133 http://orcid.org/0000-0002-7692-4133 http://orcid.org/0000-0002-7301-8274 http://orcid.org/0000-0002-7301-8274 http://orcid.org/0000-0003-0430-6093 http://orcid.org/0000-0003-0430-6093 |



Comments are closed.