Pyoderma gangrenosum in a patient with metastatic breast cancer
Nouf Bin Rubaian1,2, Amnah Al-Mulhim 3, Lenah Shaikh3
3, Lenah Shaikh3
1Dermatology Department, King Fahad Hospital of the university, Imam Abdulrahman bin Faisal University, Dammam, Saudi Arabia, 2Department of Dermatology, College of Medicine, Imam Abdulrahman bin Faisal University, Dammam, Saudi Arabia, 3Section of Dermatology, Medicine Department, King Fahad Specialist Hospital, Dammam, Saudi Arabia
Corresponding author: Amnah Al-Mulhim, MD
Submission: 19.10.2021; Acceptance: 05.04.2022
DOI: 10.7241/ourd.20223.14
Cite this article: Bin Rubaian N, Al-Mulhim A, Shaikh L. Pyoderma gangrenosum in a patient with metastatic breast cancer. Our Dermatol Online. 2022;13(3):299-301
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Pyoderma gangrenosum is a cutaneous ulcer diagnosed by exclusion. The underlining causes of pyoderma gangrenosum are numerous. Malignancy is a well-known underlying cause. Nonetheless, breast cancer is an uncommon cause, with few reported cases in the literature. There is no specific treatment of paraneoplastic pyoderma gangrenosum, apart from treating the underlying malignancy. Herein, we report a case of pyoderma gangrenosum with underlying breast cancer, successfully treated by systemic steroids, although the patient showed no response to anticancer therapy and expired shortly after the resolution of the cutaneous lesion. Further studies are needed to conclude the benefit of systemic steroids in paraneoplastic pyoderma gangrenosum, yet we recommend a trial of systemic steroids in the case of generalized resistant paraneoplastic pyoderma gangrenosum.
Key words: Pyoderma gangrenosum; Metastatic breast cancer; Systemic steroids
INTRODUCTION
Pyoderma gangrenosum is a dermatosis that presents as a painful ulcer with an undermined border. It affects females slightly more often than males, with an average age of onset of twenty to fifty years [1]. It is an idiopathic condition in 50% of cases, yet may be associated with inflammatory bowel disease, rheumatoid arthritis, and myeloproliferative disorders [1,2]. It is rarely associated with solid malignancies [2]. Herein, we report a case of extensive paraneoplastic pyoderma gangrenosum with original malignancy of the breast.
CASE REPORT
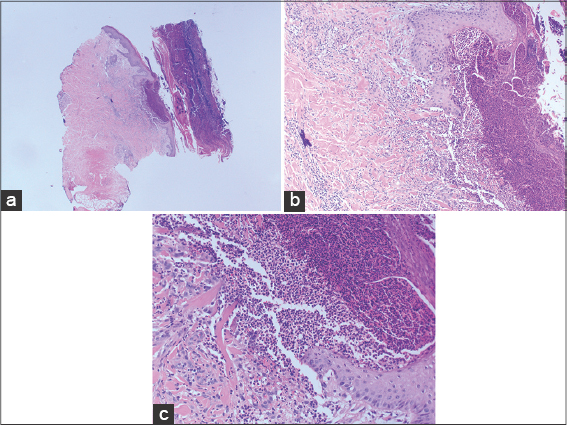
This was a 54-year-old female with type 2 diabetes, left breast cancer with metastasis to the liver, bone, and lymph nodes. She presented with a breast mass for three years, diagnosed as left breast invasive ductal carcinoma in September 2020, triple positive HER2, ER, PR on a palliative treatment of two cycles of letrozole, Herceptin, and pertuzumab. She presented with a one-month history of a single, painless papule with yellowish discharge, gradually ulcerating. Over the month, multiple similar, extensive, ulcerating lesions, ultimately healing with crustation, developed over the upper and lower extremities, buttock, and trunk. At that time, our team was consulted. Upon examination, the patient was ill-looking, cachectic, with multiple well-defined, undermined ulcers with violaceous borders and hemorrhagic necrotic crust ranging in size from several millimeters to 5 cm over the lower extremities, buttocks, back, abdomen, and arms. No lymphadenopathy was present. Swab was taken from the ulcers showing no growth. The patient initially refused biopsy and was managed with topical antibiotic ointment. One week later, the ulcers progressed and she agreed to undergo a biopsy, showing the typical features of pyoderma gangrenosum of negative PAS, GMS (Figs. 1a – 1c). A systemic steroid was discussed with the patient as an option for treatment. However, she preferred not to take the medication, worrying about a possible cancer treatment interaction. She was started on topical clobetasol propionate ointment mixed with mupirocin ointment twice daily with a fair improvement. In November 2020, the patient developed pneumonitis as a complication of the medication and was started on oral prednisolone at a dose of 1 mg/kg with the complete resolution of the ulcers.
DISCUSSION
Pyoderma gangrenosum is a neutrophilic dermatosis that classically presents as an ulcer. The exact pathophysiology is not well understood, yet it has been reported that there is a neutrophil dysfunction and overexpression of IL-8, which is a potent neutrophil chemotactic factor [1–6].
Pyoderma gangrenosum is a diagnosis of exclusion. One must exclude other causes of the ulcer before labeling the patient as a case of pyoderma gangrenosum. Cutaneous ulcers may be due to infection, vasculitis, vasculopathy, malignancies, or drugs, among other causes [4].
Inflammatory bowel disease is a common association with pyoderma gangrenosum along with rheumatoid arthritis and hematological malignancies [4,5,7].
Solid organ malignancy is not a common association, yet it has been reported. In a literature review on paraneoplastic pyoderma gangrenosum in solid organ malignancy published in September 2019 in the International Journal of Dermatology, breast cancer was the most common underlying malignancy, followed by rectal cancer. The site of the lesion was widely distrusted between the breast and lower extremities. It was thought that the tumor mediates the production of GCSF, which causes an excess of neutrophils in the blood, which eventually infiltrate the dermis and, therefore, cause a pyoderma gangrenosum lesion. Our case had no lesion on the breast, yet they were on a distant site, on the legs.
Around 80% of the cases reviewed had pyoderma gangrenosum before the diagnosis of a tumor, and it resolved spontaneously after the treatment of the tumor, yet some needed a systemic steroid [1]. However, our patient had pyoderma gangrenosum after being diagnosed with metastatic breast cancer. The patient developed the lesions while she was receiving treatment from oncology, although the lesions resolved completely only when she took prednisolone.
In one case published in the Journal of Medical Case Reports by Renata et al., pyoderma gangrenosum was found to be a recurrent condition activated by a newly diagnosed breast cancer. The initial presentation in that patient was thirty years before the breast cancer diagnosis due to her rheumatoid arthritis exacerbation. What confirms the recurrence to be associated with the malignancy is the lack of a dramatic response after prednisolone and the complete resolution after the endocrine therapy of the breast cancer as the neoplastic cells expressed the estrogen receptor [2].
Twenty-five percent of patients with pyoderma gangrenosum develop pathergy, which means PG lesions on the site of trauma. Lesions may develop after surgery, skin grafting on the donor site, and the rejection of the autologous graft on the recipient site. This was reported in a case of bilateral breast reduction with wound dehisce and infection that did not fully heal after antibiotic therapy and rejected the skin graft multiple times because of the underlying pyoderma gangrenosum. Clues to pyoderma gangrenosum in surgical cases in addition to an incomplete response to antibiotics with local wound care and autologous skin graft rejections are the pain out of proportion of the ulcer, the sparing of the areola and suture lines, worsening of the lesion with debridement, and a prompt response to immunosuppressive therapy [3–5].
CONCLUSION
Pyoderma gangrenosum may be associated with breast cancer and it should be kept in mind if the patient with breast cancer develops ulceration. It may develop even after the diagnosis of malignancy. Furthermore, if it shows no response to oncology treatment, we recommend a trial of systemic steroids.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published, and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Shah M, Sachdeva M, Gefri A, Jfri A. Paraneoplastic pyoderma gangrenosum in solid organ malignancy:A literature review. Int J Dermatol. 2020;59:154-8.
2. Duchnowska R, Ziajka E, Góralska A, Grala B. Recurrent pyoderma gangrenosum precipitated by breast cancer:A case report and review of the literature. J Med Case Rep. 2014;8:226.
3. Horner B, El-Muttardi N, Mercer D, Pyoderma gangrenosum complicating bilateral breast reduction. Br J Plast Surg. 2004;57:679-81.
4. Su WP, Davis MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum:Clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
5. Ruocco E, Sangiuliano S, Gravina AG, Miranda A, Nicoletti G. Pyoderma gangrenosum:An updated review. J Eur Acad Dermatol Venereol. 2009;23:1008-17.
6. Pandey K, Shishak M, Yadav N. Pyoderma gangrenosum:An ulcerative variant developing at a paraincisional LSCS scar site. Our Dermatol Online. 2021;2:153-5.
7. Belanouane S, Hali F, Baline K, Chiheb S, Marnissi F, Hliwa W. A rare case of malignant pyoderma associated with ulcerative colitis both treated effectively with adalimumab. Our Dermatol Online. 2020;11:393-6.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|



Comments are closed.