Clinical assessment on the efficacy of a combined treatment targeting subjects with acne-prone skin
Federica Carlomagno1, Chiara Pesciaroli, Enza Cestone2, Ileana De Ponti2, Angela Michelotti2, Francesco Tursi 2
2
1R&D Department, Roelmi HPC, Origgio (VA), 21040, Italy, 2 Complife Italia Srl, Garbagnate Milanese (MI), 20024 Italy
Corresponding author: Francesco Tursi, MD
Submission: 10.03.2022; Acceptance: 29.04.2022
DOI: 10.7241/ourd.20223.1
Cite this article: Carlomagno F, Pesciaroli C, Cestone E, De Ponti I, Michelotti A, Tursi F. Clinical assessment on the effi cacy of a combined treatment targeting subjects with acne-prone skin. Our Dermatol Online. 2022;13(3):240-247.
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Acne is a multifactorial inflammatory skin disease affecting the quality of life of acne prone subjects. Several therapeutic approaches are currently used to counteract this condition, mostly having side effects. As acne development has been recently linked to skin and gut dysbiosis, acting on both aspects could represent an alternative and promising approach to ameliorate the acne clinical signs.
Material and Methods: A cosmetic product, containing Ectoin, and a food supplement, containing probiotics, were formulated as a combined treatment to target both gut and skin microbiota and evaluated for the improvement of skin appearance on acne prone adult subjects. Eighty male and female subjects, showing acne clinical signs, were assigned to 4 groups to receive the following products: the cosmetic product containing Ectoin + a placebo food supplement, the combined treatment, a cosmetic reference product, specifically formulated for counteracting acne, + a placebo food supplement, the cosmetic reference product + the food supplement with probiotics. Acne lesions, skin sebum content, pH and moisturization were monitored.
Results: Clinical evaluations of active acne lesions and comedones, skin complexion evenness and skin inflammatory status were carried out. The combined treatment resulted as effective as the cosmetic reference product in ameliorating the instrumental parameters, and more effective in the dermatological assessment of skin complexion evenness and inflammatory status.
Conclusion: The combined treatment proposed, formulated to target both gut and skin microbiota, resulted effective in ameliorating acne clinical signs and could represent a valid alternative to conventional acne management.
Key words: acne vulgaris; dysbiosis; ectoin; microbiota; probiotics
INTRODUCTION
Skin constitutes the major organ of the body, with a main role of protection from the external environment, representing a composite ecosystem since it harbours a number of microbial communities including bacteria, fungi and viruses living in distinct niches [1]. The role of the skin microbiota is of paramount importance since it is involved in multiple host functions such as defence against pathogens, toxin degradation and host immune system maturation [2]. It seems essential, for a good functionality of such features and to maintain skin homeostasis, that communities inhabiting the skin remain in equilibrium. Indeed, it is well known that a number of skin inflammatory diseases are associated with shifts in the resident microbiota from a “healthy” to an “altered” state [3]. For example, when a stress occurs, either endogenous or exogenous, the skin ecosystem loses its equilibrium, creating conditions that directly influence the balance of skin microbiota in terms of microorganisms number and their taxonomical composition [4]. This change represents a signal for skin immunity, which reacts activating an inflammatory response. If unbalanced conditions remain for a long time and the dysbiosis persists, chronical inflammatory episodes can occur. This condition can be associated with many skin pathologies such as dandruff, acne, psoriasis and atopic dermatitis [5].
Acne is a chronic inflammatory skin disease characterized by sebum production, follicular hyperkeratinisation and inflammation, that can cause seborrhoea, inflammatory and non-inflammatory lesions and, in some cases, scarring. Although pathogenesis of this disorder is multifactorial, it is well known the important role in its onset of skin dybiosis, that may turn commensal microbiota into harmful communities [6]. In recent years, acne has also been linked to gut microbiota imbalance since an intestinal dysbiotic status can influence gut absorption by increasing its permeability, allowing toxins or inflammatory agents to reach the blood stream, leading to the development of autoimmune and inflammatory responses even in distant body districts [7].
The correlation between skin and intestine – the so-called gut–skin axis – relies on the concept that gut unbalances can affect skin by inducing systemic inflammation. Likewise, intestinal disorders are often reported in patients with skin conditions. Actually, subjects with acne prone skin result more likely to experience gastrointestinal symptoms such as constipation, abdominal bloating and gastric reflux [8]. Moreover, increased intestinal permeability causes an increment of lipopolysaccharide (LPS) endotoxins level in the blood, condition that has been observed in acne patients, triggering the activation of pro-inflammatory cytokines in sebocytes [9].
So far, common interventions to treat acne involved the topical use of retinoids, antimicrobial compounds, antibiotics and hormonal therapy. However, all these treatments can cause several reactions, from local irritation, skin drying, headache and nausea to systemic or teratogenic side effects; furthermore, the use of antibiotics can lead to the development of bacterial resistance [10]. Consequently, non-pharmacological therapies represent a viable alternative to conventional acne management. Among the new approaches investigated, restoring a healthy microbial community, by promoting the growth of symbiotic bacteria rather than only inhibiting pathogens, could be promising. Moreover, since multiple factors can be responsible for acne development, possible therapies should involve combined targets, focusing on both skin and gut microbiota [11].
In recent years, a large number of studies have explored the potential efficacy of probiotics food supplements in the prevention or treatment of dermatological disorders, due to their ability to induce positive changes into microbial population resident both in the intestinal tract and on the skin [12]. Some of these studies focused on the efficacy of oral probiotics in acne treatment, reporting their ability to reduce inflammation, by decreasing the release of inflammatory cytokines and activating regulatory T cells, and to decrease systemic levels of IGF-1, that play a role in the pathogenesis of acne [13].
Cosmetic ingredients, by contributing to maintain skin microbiota equilibrium, could represent a valid adjuvant treatment to prevent cutaneous conditions. One example is Ectoin, an amino acid derivative, firstly isolated from the bacterium Ectothiorhodospira halochloris. Its main function is to balance the salt concentration in the extracellular environment, as a protection from exogenous stress [14]. Ectoin is known for its skin osmoprotectant properties and, due to such features, is currently used for the treatment of atopic dermatitis [15]. It is also known to be effective in the treatment of inflammatory bowel disease, a condition that, amongst other factors, is characterised by dysbiosis of the intestine, by counteracting the inflammation in intestinal tissues [16]. Due to these features, this compound could act as a balancer for the skin environment, preventing microbiota dysbiosis and consequently skin inflammatory diseases, or restoring skin microbiota equilibrium when already disturbed.
Aim of the present study was to assess the effectiveness in the improvement of acne clinical signs of a novel In&Out combined acne treatment, composed by a cosmetic product containing Ectoin and a food supplement containing selected probiotic strains (L. plantarum PBS067, L. reuteri PBS072 and L. rhamnosus LRH020). In particular, the combined treatment (In&Out) was investigated for its skin improvement effect on adult subjects affected by acne through the reduction of the number and appearance of acne lesions (inflammatory and non-inflammatory acne). Evaluation of sebum level normalization and the effect on skin moisturizing and skin pH were also carried out. The effect of the In&Out treatment was compared to the effect achieved by a cosmetic reference product, targeted for acne treatment, administered with the active or placebo food supplement.
MATERIALS AND METHODS
Study Subjects
Eighty caucasian adult subjects, of both sexes, aged between 18 and 50 years old, were enrolled by a dermatologist according the following inclusion criteria: an acne severity from 1 to 3 according to IGA (Investigator’s Global Assessment) severity scale, phototype I to IV. Exclusion criteria are reported in Table 1. Written informed consent was obtained from participants before the study, including the use of non-identifiable photographs (part of the face) for publication. Regulations concerning privacy (GDPR) were observed.
 |
Table 1: Exclusion criteria |
Products
The investigated cosmetic product was a basic cream containing 1% of Ectoin, as active ingredient; the reference cosmetic formula was a finished product available on the market, specifically formulated for acne treatment.
The active food supplement (AFS) was a mixture of probiotics, in form of capsules, with the following composition: 1×109 cfu/ml of Lactiplantibacillus plantarum subsp. plantarum (formerly Lactobacillus plantarum) PBS067; 1×109 cfu/ml of Lacticaseibacillus rhamnosus (formerly Lactobacillus rhamnosus) LRH020; 1×109 cfu/ml of Limosilactobacillus reuteri (formerly Lactobacillus reuteri) PBS072 and common excipients (Maltodextrin, Magnesium stearate, Silicon dioxide, D-Biotin). The placebo food supplement (PFS) was in form of capsules and contained only excipients.
Subjects were instructed to apply a nutshell of the cosmetic/reference product in the morning on clean face and to take one capsule a day of food supplement/placebo with a glass of non-sparkling water, away from meals. No specific change in the daily habits or diet were suggested.
Study Design
A double-blind randomized placebo/reference product-controlled clinical study was carried out from May 2019 to August 2019 at Complife Italia Srl facilities in compliance with the Helsinki Declaration (1964) and its amendment. Study protocol and informed consent form were approved by the “Independent Ethical Committee for Non-Pharmacological Clinical studies” Genova, Italy (Rif. 2019/04). Study protocol was registered in the ISRCTN registry (ISRCTN18390621). Subjects were randomly assigned to 4 groups according to a randomization list previously generated by the study director using an appropriate statistic algorithm (“Wey’s urn”). The 4 groups were provided with different combinations of products as follows:
- Group 1 (G1): cosmetic product containing Ectoin + PFS
- Group 2 (G2): cosmetic product containing Ectoin + AFS (In&Out combined treatment)
- Group 3 (G3): cosmetic reference product + PFS
- Group 4 (G4): cosmetic reference product + AFS
Clinical visits were planned at baseline (T0) and after 28 (T28) and 56 (T56) days of products use.
Skin Clinical Parameters
Instrumental evaluations of the skin parameters were carried out at baseline (T0), after 28 days (T28) and 56 days (T56) of products use. Sebum level was measured by the Sebumeter® method (Sebumeter 815, Courage+Khazaka GmbH) and expressed as mg sebum/cm² of the skin. Skin moisturization was measured by the Corneometer® method (Corneometer® CM 825 (Courage+Khazaka, electronic GmbH). Skin pH was measured by SKIN pH-METER 905® (Courage + Khazaka GmbH). Dermatological assessment was carried out by a dermatologist by counting acne lesions in terms of number of papules, pustoles, open comedones (blackheads) and closed comedones (whiteheads). Clinical classification of skin complexion evenness was carried out at baseline, after 28 and 56 days of treatment by assigning a variation score with respect to the basal classification. Criteria used are reported in Table 2.
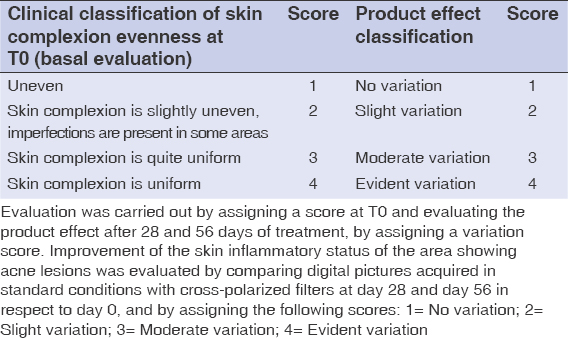 |
Table 2: Clinical and product effect classification |
Digital pictures of the subjects face were acquired at each experimental time using a reflex digital camera (NIKON D300 digital camera) equipped with macro-objective (AF-S Micro NIKKOR 60mm f/2.8G ED), a flash system (Kit R1C1) and cross-/parallel-polarized filters, all from Nikon Corporation Tokyo, Japan.
Statistical Analysis
Instrumental data were submitted to ANOVA test followed by Tukey-Kramer post-test (intra-group analysis); the inter-group statistical analysis was performed on the data variations versus T0 by means of Bilateral Student’s Test t for unpaired data. Clinical data were analysed using Mann-Whitney U/Wilcoxon Rank-Sum Test (Two Samples). Statistical analysis was performed using NCSS 10 statistical software (NCSS, LLC. Kaysville, Utah, USA) running on Windows Server 2008 R2 Standard (Microsoft, USA).
RESULTS
All subjects (17 males and 63 females, with an average age of 28.0±9.0) completed the study; treatments were well tolerated and no adverse events were reported.
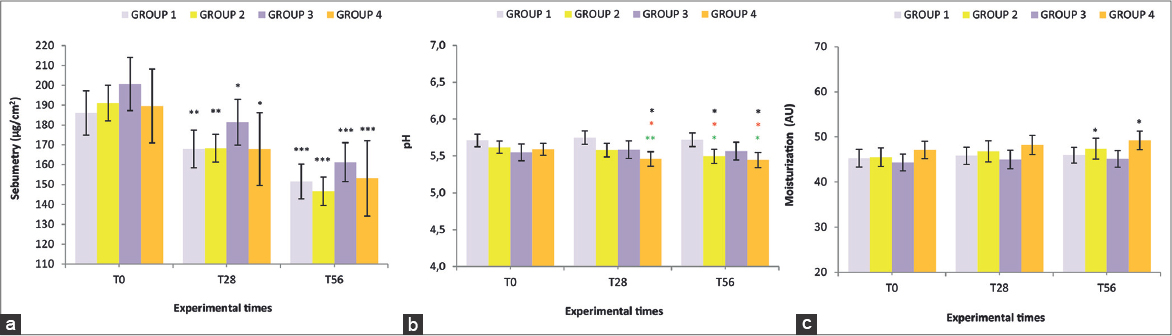
All 4 groups showed statistically significant improvements in almost all the parameters studied compared to T0. Sebum basal levels did not show any significant intergroup difference, supporting the homogeneity of the groups (Fig. 1a). A significant decrease of this parameter, with respect to T0, was recorded in all the 4 groups already at T28 (p<0.05 for G3 and G4, p<0.01 for G1 and G2). Reduction resulted even more evident at T56 (p<0.001 for all the groups) with the highest level achieved by G2 (-22%) and G4 (-20.7%). No intergroup significant differences were observed. The homogeneity of the panel was also confirmed for the skin pH (Fig. 1b), that showed a statistically significant diminution with respect to the basal level in G2 at T56 (p<0.05) and in G4 at T28 and at T56 (p<0.05 for both). Furthermore, significant intergroup differences were also observed. As shown in Fig. 1c, basal levels of hydration did not differ in the four groups. All treatments induced an increment of such parameter, which achieved, at T56, a significant increase (p<0.05), with respect to baseline, for G2 (+3,9%) and G4 (+4,7%). No intergroup significant differences were observed.
Basal number of inflammatory acneic lesions were almost the same in all the groups (Fig. 2a). All treatments achieved a reduction with respect to the beginning of the study, that resulted statistically significant already at T28 (p<0.05 for G1, G2 and G3; p<0.001 for G4) and further improved at T56 (p<0.01 for G1; p<0.001 for all the other groups). The highest levels of percentage reduction were observed in G2 (-4,4%) and G4 (-4.0%). No intergroup significant differences were observed. As for the number of comedones (Fig. 2b), all the tested treatments achieved a progressive diminution that resulted statistically significant at T56 with respect to baseline for all the groups (p<0.01 for G3; p<0.001 for the other groups).
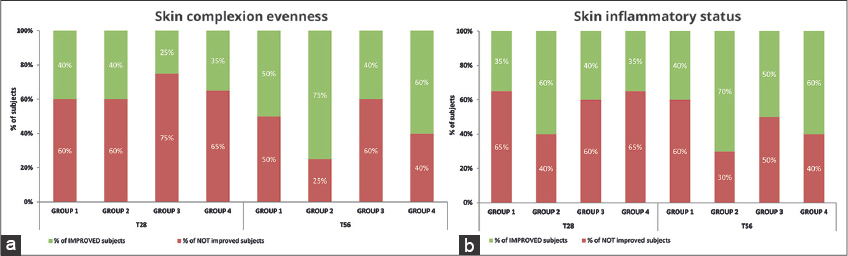
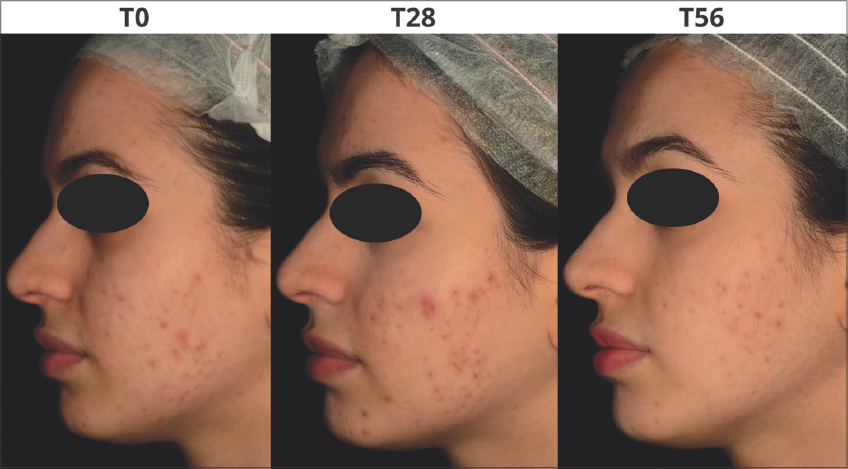
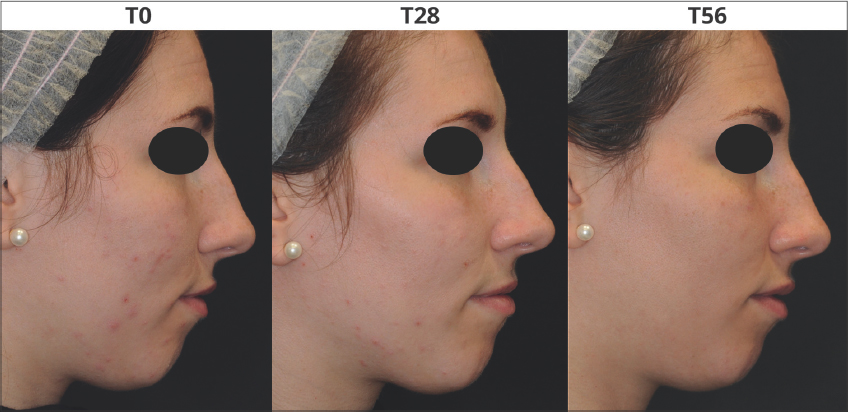
Dermatological assessment showed statistically significant improvements by the In&amp;amp;amp;amp;Out treatment compared to the cosmetic treatment alone. An improvement of the skin complexion evenness was recorded in all groups. The intragroup differences resulted more relevant after 56 days as G2 and G4 showed an amelioration of the initial scores by 75% and 60% respectively (Fig. 3a). Also for the skin inflammatory status of the area showing acne lesions, a remarkable improvement was recorded in all groups; the intragroup difference resulted more relevant in G2 and G4 that, after 56 days of treatments, showed an amelioration of the initial scores by 70% and 60% respectively (Fig. 3b). Figs. 4 and 5 are a set of representative digital pictures showing the progressive improvement achieved in G2 and G4 groups throughout the study.
DISCUSSION
Acne is a multifactorial skin disease and its complex pathophysiology has been recently linked to skin and gut dysbiosis [6,7]. Indeed, there is growing awareness that maintaining a balanced skin environment and counteracting skin dysbiosis, is the first step for the health of skin microbial communities and, consequently, to avoid the onset of cutaneous diseases [17]. It has also been reported that the functional integrity of intestinal tract microbial residents may play a mediating role in skin inflammation [18]. Therefore, acting on the equilibrium of both the intestinal and the cutaneous microbiota represents an interesting approach to be implemented in the daily routine with respect to the known most aggressive treatments for acute phase.
This double-blind randomized placebo/reference product-controlled clinical study evaluated the efficacy, in adult subjects affected by active acne, of a novel approach based on a combined cosmetic and nutraceutical treatment (In&amp;amp;amp;amp;Out): a cosmetic product containing Ectoin as active ingredient and a food supplement containing selected probiotics. Both cosmetic and nutraceutical ingredients were chosen according results obtained in other studies. As for the Ectoin, in-vitro tests, carried out on human skin keratinocytes, highlighted its capability to maintain cell homeostasis (cell viability and cell metabolism) after osmostress induction and to enhance surface expression of b-Defensin 1 in cells treated with LPS, resulting in a protective effect on skin. Furthermore, a clinical placebo-controlled trial on women exposed to adverse environmental conditions (high level of pollution) showed a remarkable effect of Ectoin on skin moisturization, elasticity, general skin profilometry and, according to metagenomic analysis, in maintaining a more balanced skin microbiome compared to placebo [19]. The probiotic strains showed significant in-vitro ability in the modulation of inflammatory status and antimicrobial activity against skin pathogens such as C. acnes, S. epidermidis (data not shown) and S. aureus [20]. Furthermore, the same probiotic formulation resulted effective in a randomized double-blind placebo-controlled clinical trial on adult subjects showing mild to severe atopic dermatitis, ameliorating skin clinical parameters (smoothness, moisturization and SCORAD index) and decreasing levels of skin inflammatory markers [21].
Results obtained in this study were highly promising considering that the combined approach was assayed also with a dermocosmetic benchmark already on the market and specifically formulated for acne, thus containing targeted active dermatological ingredients (alpha-hydroxy acids esters, citric and salicylic acids, zinc etc.) reported to be effective on abnormal keratinization, inflammation and to have antimicrobial activity [22].
All 4 groups showed significant improvements of sebum levels compared to T0 after 28 and 56 days and also a significant acneic lesion reduction by the end of the treatment, suggesting a direct effect on acne. G2 (In&amp;amp;amp;amp;Out) and G4 (benchmark+probiotics), showed better results compared to G1 and G3, i.e. treatments without the probiotics, as well as a statistically significant improvement of both skin pH and skin hydration after 56 days, supporting the hypothesis that a combined treatment could be an innovative approach to investigate in order to achieve acne amelioration.
Interestingly, G2 always showed a similar efficacy in all the instrumental parameters tested compared to G3, that represents the conventional dermocosmetic approach to acne, moreover the improvement achieved by G2 at the end of the study period were higher than the benchmark product alone (G3) both in the clinical assessment of skin complexion evenness (75% vs. 40%, respectively) and in the skin inflammatory status of the area interested by acne lesions (70% vs. 50%, respectively). Altogether, such results suggest that the probiotic intake can have a role in the gut-skin axis, helping in the restore of skin barrier in a more efficient way than the cosmetic alone. Indeed, the effectiveness of the probiotic formulation was confirmed by the improvement of acne symptoms achieved in the G4 group with respect to the benchmark alone. It is worth noting that the cosmetic containing Ectoin was specifically formulated to restore an unbalanced skin microbiota and not focused on acne treatment: on this basis the fact that results obtained were almost similar to the benchmark is significant and innovative.
The main objective of this clinical trial was to assess the efficacy of a treatment based on the restoring of the microbiota equilibrium both at skin and gut level since the disruption of the delicate balance between the host and the resident microorganisms is nowadays considered responsible for many skin distresses [5]. For example, the increasing growth of Cutibacterium acnes, a common member of the skin microbiota, has been linked to the pro-inflammatory response in the follicle and in the adjacent dermis, leading to acne onset [6]. Conventional treatments against its proliferation involve the use of antibiotics and/or compounds that have significant concomitant reactions. Indeed, commonly used therapies for inflammation caused by acne include strong anti-inflammatory systemic drugs that can generate dry skin, erythema, desquamation etc. In this context, a cosmetic ingredient such as Ectoin, that helps to maintain skin equilibrium without side effects, could be a valuable option. Likewise, probiotics have been demonstrated to help reducing the inflammatory cytokines cascade by several mechanisms such as upregulation of Treg cells and modulation of Th1 and Th2 response, and represent a therapeutic option without potential adverse events typical of chronic antibiotic use. The strains used in the food supplement have previously shown to reduce not only skin inflammation markers but also those related to the systemic inflammation [21,23]. Furthermore, they have been reported to successfully colonize the GI tract, showing long term performance in the reduction of skin condition symptoms [21,24]. This is particularly interesting considering the cross talk between the gut and the skin, where probiotics are responsible both for the production of beneficial metabolites that can reach the skin and for their antimicrobial activity through the release of bacteriocins, as reported for the strain L. plantarum PBS067 [25].
CONCLUSIONS
The combined treatment proposed ameliorates clinical acneic signs with a good tolerability, demonstrating that targeting both the gut and the skin microbiota could be a valid adjuvant therapy for the management of acne, and suggesting that such approach could represent a promising alternative to the current pharmacological therapy of chronic skin conditions.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study, including the use of non-identifiable photographs (part of the face), was obtained from all patients.
REFERENCES
1. Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244-53.
2. Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506.
3. Balato A, Cacciapuoti S, Di Caprio R, Marasca C, MasaràA, Raimondo A, et al. Human microbiome:composition and role in inflammatory skin diseases. Arch Immunol Ther Exp. 2019;67:1-18.
4. Morvan PY, Vallee R. Evaluation of the effects of stressful life on human skin microbiota. Appli Microbiol Open Access. 2018;4:2.
5. Chen P, He G, Qian J, Zhan Y, Xiao R. Potential role of the skin microbiota in inflammatory skin diseases. J Cosmet Dermatol. 2021;;20:400-9.
6. Ramasamy S, Barnard E, Dawson TL Jr, Li H. The role of the skin microbiota in acne pathophysiology. Br J Dermatol. 2019;181:691-9.
7. Yan HM, Zhao HJ, Guo DY, Zhu PQ, Zhang CL, Jiang W. Gut microbiota alterations in moderate to severe acne vulgaris patients. J Dermatol. 2018;45:1166-71.
8. Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis:from anecdote to translational medicine. Benef Microbes. 2014;5:185-99.
9. Dréno B, Dagnelie MA, Khammari A, Corvec S. The skin microbiome:a new actor in inflammatory acne. Am J Clin Dermatol. 2020;Sep;21(Suppl 1):18-24.
10. Walocko FM, Eber AE, Keri JE, Al-harbi MA, Nouri K. The role of nicotinamide in acne treatment. Dermatol Ther. 2017;Sep;30(5).
11. Lee YB, Byun EJ, Kim HS. Potential Role of the Microbiome in Acne:A Comprehensive Review. J Clin Med. 2019;8:987.
12. SzántóM, Dózsa A, Antal D, SzabóK, Kemény L, Bai P. Targeting the gut-skin axis -Probiotics as new tools for skin disorder management. Exp Dermatol. 2019;28:1210-8.
13. Kober MM, Bowe WP. The effect of probiotics on immune regulation, acne, and photoaging. Int J Womens Dermatol. 2015;1:85-9.
14. Galinski EA, Pfeiffer HP, Trüper, HG. 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid:A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur J Biochem. 1985;149:135-9.
15. Marini A, Reinelt K, Krutmann J, Bilstein A. Ectoine-containing cream in the treatment of mild to moderate atopic dermatitis:a randomised, comparator-controlled, intra-individual double-blind, multi-center trial. Skin Pharmacol Physiol. 2014;27:57-65.
16. Abdel-Aziz H, Wadie W, Abdallah DM, Lentzen G, Khayyal MT. Novel effects of ectoine, a bacteria-derived natural tetrahydropyrimidine, in experimental colitis. Phytomedicine. 2013;20:585-91.
17. Prescott SL, Larcombe DL, Logan AC, West C, Burks W, Caraballo L, et al. The skin microbiome:impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ J. 2017;10:29.
18. Bowe WP, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis – back to the future. Gut Pathog. 2011;3:1.
19. Carlomagno F, Zanzottera S. Empowering the Micro-World of the Skin Microbiota:Approaches to Maintain Nature’s Ideal Homeostasis. Eurocosmetics. 2019;6.
20. Presti I, D’Orazio G, Labra M, La Ferla B, Mezzasalma V, Bizzaro G, et al. Evaluation of the probiotic properties of new Lactobacillus and Bifidobacterium strains and their in vitro effect. Appl Microbiol Biotechnol. 2015;99:5613-26.
21. Michelotti A, Cestone E, De Ponti I, Giardina S, Pisati M, SpartàE, et al. Efficacy of a probiotic supplement in patients with atopic dermatitis:a randomized, double-blind, placebo-controlled clinical trial. Eur J Dermatol. 2021;31:225-32.
22. Araviiskaia E, Dréno B. The role of topical dermocosmetics in acne vulgaris. J Eur Acad Dermatol Venereol. 2016;30:926-35.
23. Cicero AFG, Fogacci F, Bove M, Giovannini M, Borghi C. Impact of a short-term synbiotic supplementation on metabolic syndrome and systemic inflammation in elderly patients:a randomized placebo-controlled clinical trial. Eur J Nutr. 2021;60:655-63.
24. Mezzasalma V, Manfrini E, Ferri E, Sandionigi A, La Ferla B, Schiano I, et al. A Randomized, Double-Blind, Placebo-Controlled Trial:The Efficacy of Multispecies Probiotic Supplementation in Alleviating Symptoms of Irritable Bowel Syndrome Associated with Constipation. Biomed Res Int. 2016;2016:4740907.
25. De Giani A, Bovio F, Forcella M, Fusi P, Sello G, Di Gennaro P. Identification of a bacteriocin-like compound from Lactobacillus plantarum with antimicrobial activity and effects on normal and cancerogenic human intestinal cells. AMB Express. 2019;17;9:88.
Notes
Source of Support: Source of Support: This study was realized in the frame of SCIDA project (Study and development of new pro- and prebiotic products for prevention and treatment of inflammatory diseases such as irritable bowel syndrome and Atopic dermatitis) funded by Lombardy Region [2014IT16RFOP012-POR FESR 2014-2020-Project ID226149].
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-9911-7094 http://orcid.org/0000-0002-9911-7094 http://orcid.org/0000-0002-5497-5036 http://orcid.org/0000-0002-5497-5036 http://orcid.org/0000-0002-2541-6139 http://orcid.org/0000-0002-2541-6139 http://orcid.org/0000-0003-0579-7904 http://orcid.org/0000-0003-0579-7904 http://orcid.org/0000-0001-5249-9913 http://orcid.org/0000-0001-5249-9913 http://orcid.org/0000-0002-0055-5925 http://orcid.org/0000-0002-0055-5925 |




Comments are closed.