Active vitiligo vulgaris following the administration of the Oxford–AstraZeneca (AZD1222) vaccine against SARS-CoV-2
Ahmed Abdul-Aziz Ahmed 1, Ghusoon Nazar Jaber2
1, Ghusoon Nazar Jaber2
1Department of Dermatology, College of Medicine, Tikrit University, Iraq, 2AL Ramadi Teaching Hospital, Department of Dermatology, Iraq
Corresponding author: Ahmed Abdul-Aziz Ahmed, MD, FICMS
How to cite this article: Abdul-Aziz Ahmed A, Jaber GN. Active vitiligo vulgaris following the administration of the Oxford–AstraZeneca (AZD1222) vaccine against SARS-CoV-2. Our Dermatol Online. 2022;13(2):219-220.
Submission: 29.10.2021; Acceptance: 05.01.2022
DOI: 10.7241/ourd.20222.27
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
Sir,
There is significant uncertainty surrounding vaccines against COVID-19. Due to the critical need, these vaccines are being developed and approved at a uniquely fast pace [1]. Currently, the approved vaccines take their action by administering the host with sequences encoding the viral spike protein. This essentially means that these gene-therapy-based vaccines, whose long-term effects remain unknown, are going to be administered globally [2]. Therefore, despite their safety and efficacy demonstrated in respective clinical trials, it is reasonable to be cautious and adopt more intensive post-marketing vigilance [3].
A forty-nine-year-old diabetic male presented with asymptomatic, rapidly growing, leukodermal patches on the scalp (Fig. 1), which began to appear suddenly fourteen days following the administration of the first dose of the Oxford–AstraZeneca (AZD1222) vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The patches were clinically consistent with vitiligo and were confirmed with their well-defined, ivory-white patches by Wood’s lamp examination (Fig. 2).
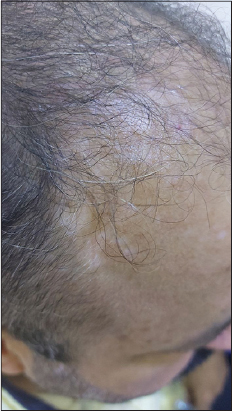 |
Figure 1: Vitiligo on the right side of the scalp. |
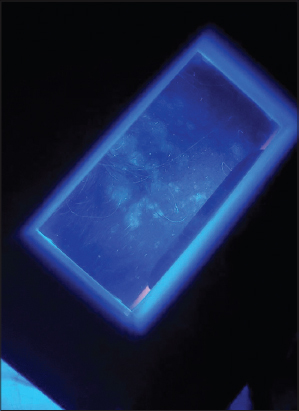 |
Figure 2: Vitiligo confirmed with their well-defined, ivory-white patches. |
There were no leucodermic patches anywhere else on the body, no family history of vitiligo, and no history of COVID-19 viral infection. After one week of reevaluation, the patch increased in size. The patient also presented with cellulitis on the right groin persistent for three days and was treated with an antibiotic with a follow-up. All baseline investigations, including a full blood count and renal and liver function tests, were normal. The patient is currently on follow-up.
Cytokines play a role in regulating the immune response and the depigmentation process in vitiligo. There is an imbalance in cytokine levels in patients with vitiligo. IFN-g expression plays a role in the autoimmune process in vitiligo. The expression of the cytokine IFN-g is associated with melanocyte destruction in the active phase of vitiligo lesions [4].
IFN-g interacts with the viral receptor, resulting in the consequent reduction of several virus replicating, down-regulating genes and gene products [5]. Li et al. [6] demonstrated that IFNs are potential drug choices for SARS-CoV-2 infection.
Here, we hypothesize that patients with non-segmental vitiligo (NSV), an autoimmune skin (and mucosal) disorder, may clear SARS-CoV-2 infection more efficiently and have a lower risk of COVID-19 development. Conversely, in the case of COVID-19 development, vitiligo autoimmunity may influence the cytokine storm-related disease burden. In addition, immune activation during SARS-CoV-2 infection or COVID-19 disease may increase vitiligo disease activity. Our hypothesis is based on the shift of the immune system in NSV toward adaptive type 1 (IFNg and CD8 T cells) and innate immune responses [7].
It is unclear whether the vitiligo in our patient was caused by vaccination, However, the temporal relationship between the vaccine and the development of the disease is interesting. We may, therefore, conclude that our patient had his latent vitiligo activated by the vaccine. Yet, further work is needed to demonstrate a causal relationship between vitiligo and COVID-19 vaccination.
To the best of our knowledge, this is the first reported case of vitiligo associated with the administration of the Oxford–AstraZeneca (AZD1222) vaccine.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Rosengard HC, Wheat CM, Tilson MP, Cuda JD. Lichen planus following tetanus–diphtheria–acellular pertussis vaccination:A case report and review of the literature. SAGE Open Med Case Rep. 2018;6:2050313X1775033.
2. Koven S. Developing COVID-19 vaccines at pandemic speed. N Engl J Med. 2020;28:1-2.
3. Sinha A, Kumar R, Singh AR. Implication of mass COVID-19 vaccination on dermatology practice in 2021. Dermatol Ther. 2021;e14765.
4. Ezzedine S, El Rewiny EM, Abozied AA, Samna SM. Evaluation of serum interferon-gamma level in vitiligo patients. Egypt J Hosp Med. 2018;73:7806-13.
5. Levy DE, García-Sastre A. The virus battles:IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev. 2001;12:143-56.
6. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020;19:149-50.
7. Post NF, Luiten RM, Wolkerstorfer A, Bekkenk M, Böhm M. Does autoimmune vitiligo protect against COVID-19 disease?Exp Dermatol. 2021;30:1254-7.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-3995-4373 http://orcid.org/0000-0003-3995-4373 |



Comments are closed.