Chronic scrotal erythredema revealing lupus in a sixteen-year-old male
Meriem Elmansouri , Fatima-Zohra Elfatoiki, Fouzia Hali, Soumiya Chiheb
, Fatima-Zohra Elfatoiki, Fouzia Hali, Soumiya Chiheb
Department of Dermatology and Venereology, HUC Ibn Rochd, University Hassan II of Casablanca, Morocco
Corresponding author: Meriem Elmansouri, MD
How to cite this article: Elmansouri M, Elfatoiki FZ, Hali F, Chiheb S. Chronic scrotal erythredema revealing lupus in a sixteen-year-old male. Our Dermatol Online. 2022;13(2):168-171.
Submission: 23.02.2021; Acceptance: 16.05.2021
DOI: 10.7241/ourd.20222.12
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
The literature insufficiently describes the etiologies of chronic penoscrotal edema, and even more so those of chronic permanent scrotal erythredema. Herein, we report the case of a sixteen-year-old male who presented, in addition to permanent and chronic scrotal erythredema, classical cutaneous manifestations of systemic lupus. The patient had received systemic corticosteroid therapy combined with hydroxychloroquine. The evolution was good, without new outbreaks or recurrences of penoscrotal edema. Apart from the usual urological emergencies, systemic diseases may be involved in scrotal erythredema, which requires long-term medical management.
Key words: Scrotal erythredema; Systemic lupus erythematosus; Male genitalia
INTRODUCTION
Penoscrotal edema is a frequent pediatric emergency associated with multiple etiologies. Urological emergencies predominate and should always be investigated. However, some scrotal edemas may be of systemic origin. Herein, we report the case of a sixteen-year-old male who presented classic cutaneous–systemic manifestations of systemic lupus associated with permanent and chronic scrotal erythredema. The latter is an uncommon manifestation of cutaneous lupus.
CASE REPORT
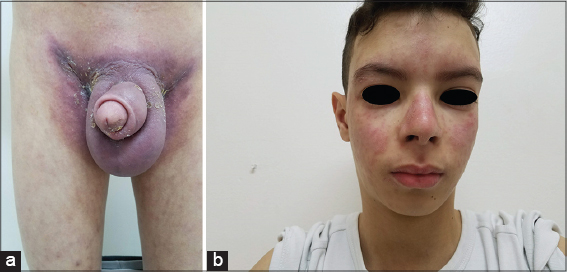
A sixteen-year-old male presented with permanent scrotal erythredema persistent for three months. The patient presented himself six months earlier with painful nasal ulcerations, alopecic scalp lesions, and photosensitivity. The symptomatologic evolution was marked three months later by the sudden onset of inflammatory polyarthralgia and painless scrotal erythredema, which gradually worsened and was associated with maculopapular face lesions, fever, and weakness. A physical examination revealed a fever at 39°C and purplish scrotal erythredema, which extended to the perineal and inguinal areas (Fig. 1a). Testicular bursa palpation revealed no evidence of isolated scrotal lymphedema. The patient showed a maculopapular erythematous malar rash with a butterfly distribution extending to the forehead and respecting the nasolabial folds (Fig. 1b). Several discoid lesions were present in the arms with small alopecic scalp plaques. The patient also had a permanent reticular livedo of the lower limbs (Fig. 1a) with multiple cervical and inguinal lymphadenopathy.
Doppler ultrasound of the scrotum highlighted scrotal wall thickening and subcutaneous tissue edema with normal testicles and epididymis. Laboratory data included normochromic normocytic anemia with the hemoglobin level at 6.9 g/dL (12–18) and the rate of erythrocyte sedimentation slightly increased, at 40 mm (N < 20). Immunological samples revealed a speckled antinuclear antibody (ANA) pattern at a titer > 1.280 and positive anti-Sm antibodies with negative anti-DNA. The direct Coombs test was positive. C3 and C4 complement levels were within normal values. There was no hematuria or proteinuria. An infectious disease workup for syphilis, HIV, hepatitis B and C, and tuberculosis was negative. A chest x-ray, abdominopelvic ultrasound, and transthoracic echocardiography were normal. On histological examination of a perineal fragment, the epidermis was hyperacanthotic, papillomatous, and hyperorthokeratotic without interface involvement. The dermis was edematous with ectatic vessels in the superficial and middle dermis. Perivascular lymphocytic infiltration was minimal. Histology of the inguinal lymph nodes suggested a reactive infiltrate without signs of malignancy.
The mucocutaneous manifestations, the cervical and inguinal lymphadenopathy with fever, the hematological manifestations, and the high titers of ANA and SM antibody associated with a positive direct Coombs test were all consistent with the diagnosis of SLE. The patient received pulsed steroid treatment for three days, followed by oral prednisolone (2 mg/kg/day) and hydroxychloroquine (4 mg/kg/day) with external photoprotection.
The short-term outcome was favorable, characterized by a general clinical improvement and decreased clinical and laboratory signs, including scrotal erythredema. The systemic corticosteroid therapy was gradually reduced after two years, without any new relapse or recurrence of penoscrotal edema. Regular systematization assessments were always negative (Fig. 2).
 |
Figure 2: Clinical improvement with the disappearance of the scrotal erythredema. |
DISCUSSION
Scrotal edema may affect the dermis and/or subcutaneous tissue as well as the contents of the scrotum [1]. The origin of the scrotal edema may easily be elucidated by well-performed clinical and paraclinical examinations [2]. Its management is most often surgical. However, in some situations, diagnosis is not evident. Chronic penoscrotal edema etiologies and chronic permanent scrotal erythredema have insufficiently been reviewed in the literature [3].
A wide range of etiologies are associated with chronic scrotal edema. They may be general (heart failure, kidney disease, and ascites), obstructive (abdominal neoplasms, inguinal hernias, and spermatic vein thrombosis), infectious (filarial elephantiasis), iatrogenic (radiotherapy, lymph node dissection), and traumatic [1,4]. Inflammatory affection may be secondary to these situations and, therefore, be responsible for scrotal erythematous edema [5]. In addition, various inflammatory pathologies may affect skin coating and be responsible for scrotal edema. Among these etiologies, we find fungal skin infections, hidradenitis suppurativa, reverse psoriasis, Behçet’s disease, and extramammary Paget disease [5]. In our patient, a clinical examination of the container and scrotal contents, combined with the results of scrotal imaging and histology, allowed us to exclude all the aforementioned etiologies.
Erythematous scrotal edema or scrotal erythredema is an infrequently described entity. Few cases of scrotal erythredema associated with systemic diseases have been reported in the literature, particularly with Crohn’s disease [6], Henoch–Schönlein purpura, sarcoidosis [7], rheumatoid arthritis [8], juvenile dermatomyositis [9], testicular vasculitis [10], non-Hodgkin’s lymphoma, and Kaposi’s sarcoma [8]. However, our patient’s clinical status did not meet any of the above-mentioned etiologies. In contrast, apart from scrotal erythredema, all of our patient’s clinical, biological, and immunological manifestations met the SLICC criteria for systemic lupus erythematosus (SLE) [11,12].
15% to 20% of patients with SLE develop signs and symptoms during childhood and adolescence [11]. Juvenile systemic lupus erythematosus (JSL) is suggested if SLE develops before the age of eighteen [13]. Mucocutaneous manifestations are the second most frequent affection in SLE-J after hematological affection [14]. Several types of skin lesions are noticed during the course of SLE. Lupus lesions are found, defined by their clinical, histological, and progressive appearance, and non-lupus, vascular or non-vascular manifestations, are mainly present in systemic forms [15,16]. Also, atypical and infrequent skin show have been reported [17,18]. Systemic lupus skin lesions usually occur in light-exposed areas. On the other hand, genital localization is rare and is poorly documented in the literature, hence the interest of our case [19,20].
Inuzuka et al. reported the case of a 71-year-old Japanese female who had infiltrated erythematous patches on the eyelids and subcutaneous nodules on the hands, thigh, and leg. She also suffered from mouth ulcers, arthralgias, and fever. Laboratory tests revealed elevated antinuclear antibodies, an increased rate of erythrocyte sedimentation, and anemia. Skin biopsies from the hand and thigh showed perivascular lymphocytic infiltrates as well as vacuolar changes in the basement membrane of the appendices. In addition, there was a dense lymphocytic infiltrate in the dermis with extension into the subcutaneous fat, which was consistent with the diagnosis of deep lupus erythematosus. Although a biopsy from an eyelid lesion does not contain subcutaneous fat, the changes in the dermis are essentially the same as those in the hand and thigh. The rash and other symptoms disappeared quickly with oral prednisolone [17]. In our patient, except for the genital localization and unspecific histological results, the clinical–biological status was superimposable. Other similar cases have been reported in a systematic review by Mullaaziz et al., with systemic signs such as arthritis and fever and with abnormal biological and immunological assessments, as in our patient [18]. By analogy with this association, “palpebral edema and systemic lupus,” we linked our patient’s scrotal edema to systemic lupus. This was based on clinical, biological, and especially evolutionary arguments in the face of the disappearance of the scrotal erythredema after treating the lupus with a two-year follow-up.
CONCLUSION
Scrotal erythredema is rarely described. Apart from the usual urological emergencies, systemic diseases may be responsible for this genital manifestation. The knowledge of this origin is important because the treatment is medical and involves long-term care.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Weinberger L, Zirwas M, English III J. A diagnostic algorithm for male genital oedema. J Eur Acad Dermatol Venereol. 2007;21:156-62.
2. Gratzke C, Seitz M, Zaak P, Reich O, Schlenker b, Stief CG. [Painless enlargement of the scrotum]. MMW Fortschr Med. 2006;148:42-5.
3. Sehgal VN, Sehgal R, Sehgal D, Pandey SS, Amin SS, Bhattacharya SN, et al. Scrotal swellings synopsis of differential diagnosis (Part III). Invest Dermatol Venereol Res. 2016;2:84-90.
4. Rajkumar PN, Venukumar KN, Dinesh MG, Satishbabu N. Diagnosis and treatment of scrotal swelling in adults:Our experience. Jebmh. 2016;3:1503.
5. Ebert AK. [Pathological findings in the scrotum and testis and initial diagnostic management]. Urologe A. 2010;49:1476-80,1482-4.
6. Rajah K, Oliver MR, McLeod L, Orchard D, Leal M. Unusual manifestations of a common gastrointestinal disorder:Instructive case. J Paediatr Child Health. 2014;50:158-60.
7. Kimura S, Momozono K, Shimamatsu K, Noguchi M. Testicular sarcoidosis with bilateral scrotal swelling. IJU Case Rep. 2020;3:12-4.
8. Dudley AG, Fox JA, Reyes-Múgica M, Cannon G. Penoscrotal edema and purpura in a 12-year-old boy:A case report and review of causes. J Pediatr Urol. 2012;8:e47-50.
9. Sallum AME, Silva MFC, Michelin CM, Duarte RJ, Baroni RH, Aikawa NE, et al. Penile and scrotum swelling in juvenile dermatomyositis. Acta Reumatol Port. 2011;36:176-9.
10. Lintern N, Johnson NR, Mckenzie I, Martin B. Testicular vasculitis –Literature review and case report in queensland. Curr Urol. 2013;7:107-9.
11. Chiewchengchol D, Murphy R, Edwards SW, Beresford MW. Mucocutaneous manifestations in juvenile-onset systemic lupus erythematosus:A review of literature. Pediatr Rheumatol. 2015;13:1.
12. Hiraki LT, Benseler SM, Tyrrell PN, Hebert D, Harvey E, Silverman ED. Clinical and laboratory characteristics and long-term outcome of pediatric systemic lupus erythematosus:A longitudinal study. J Pediatr. 2008;152:550-6.
13. Smith EMD, Lythgoe H, Midgley A, Beresford MW, Hedrich CM. Juvenile-onset systemic lupus erythematosus:Update on clinical presentation, pathophysiology and treatment options. Clin Immunol. 2019;209:108274.
14. Levy DM, Kamphuis S. Systemic lupus erythematosus in children and adolescents. Pediatr Clin North Am. 2012;59:345-64.
15. Chiewchengchol D, Murphy R, Morgan T, Edwards SW, Leone V, Friswell M, et al. Mucocutaneous manifestations in a UK national cohort of juvenile-onset systemic lupus erythematosus patients. Rheumatology (Oxford). 2014;53:1504-12.
16. Abreu-Velez AM, Smoller BR, Howard MS. Lesional IgE in flares induced by drugs in a patient with autoimmune lupus erythematosus. Our Dermatol Online. 2020;11:260-3.
17. Inuzuka M, Tomita K, Tokura Y, Takigawa M. Lupus erythematosus profundus with unusual skin manifestation:Subcutaneous nodules coexisting with eyelid plaques. J Dermatol. 2001;28:437-41.
18. Mullaaziz D, Maden S, Deren O, Özkayalar H. Periorbital lupus erythematosus profundus:A case report and review of the literature. TURKDERM. 2020;54:114-6.
19. Bailey BM, Ramos KS, Johnson A, Mitchell C. Acute hepatic failure and epididymitis in a Hispanic patient with active systemic lupus erythematosus. J Clin Med Res. 2018;10:722-4.
20. Romiti R, Anzai A, Nico M. Genital discoid lupus:A rare manifestation of cutaneous lupus erythematosus. Lupus. 2014;23:707-10.
Notes
Source of Support: Nil,
Conflict of Interest: None
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-9475-2785 http://orcid.org/0000-0002-9475-2785 |



Comments are closed.