Refractory pemphigus vulgaris with extrapulmonary tuberculosis: A challenging case management with rituximab
Munishamappa Ramesh, Abhineetha Hosthota , Thejaswi R Sanjay
, Thejaswi R Sanjay
Department of Dermatology, Venereology & Leprosy, The Oxford Medical College, Hospital & Research Center, Yadavanahalli, Bangalore, India
Corresponding author: Abhineetha Hosthota, MD
How to cite this article: Ramesh M, Hosthota A, Sanjay TR. Refractory pemphigus vulgaris with extrapulmonary tuberculosis: A challenging case management with rituximab. Our Dermatol Online. 2022;13(2):165-167.
Submission: 16.05.2021; Acceptance: 09.10.2021
DOI: 10.7241/ourd.20222.11
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Pemphigus vulgaris is an autoimmune acantholytic blistering disease that affects the skin and mucous membranes and that is due to IgG autoantibodies targeted against adhesion molecules such as desmoglein 1 and 3 in the epidermis. It follows a chronic course with remissions and exacerbations affecting the patient’s quality of life. Since the advent of immunosuppressants, the prognosis of pemphigus has improved but mortality still persists. Long-term use of high-dose corticosteroids and immunosuppressants leads to serious adverse events. Recently, rituximab, a chimeric anti-CD20 monoclonal antibody, which causes B-cell depletion, has been shown to improve disease remission rates with faster tapering of steroids compared to the conventional treatment. Herein, we present a case of refractory pemphigus vulgaris with extrapulmonary tuberculosis and myelosuppression, focusing on unmet challenges due to high-dose systemic steroids, azathioprine, and successful management of the case with rituximab.
Key words: Pemphigus vulgaris; Rituximab; Extrapulmonary tuberculosis
INTRODUCTION
Pemphigus vulgaris (PV) is an autoimmune bullous disorder caused by circulating autoantibodies directed against desmogleins, resulting in the loss of cell–cell adhesion characterized by blistering of the skin and mucosa. The therapeutic armamentarium includes immunosuppressants such as corticosteroids, azathioprine, mycophenolates, and immunomodulators such as dapsone and tetracyclines. Prolonged and high-dose administration of immunosuppressants is often required to control PV [1].
Some immunocompromised patients are at risk of acquiring primary tuberculosis (TB) or the reactivation of non-active tuberculosis [1]. It is the second leading cause of death from an infectious disease (after HIV infection) [2]. According to the WHO classification, extrapulmonary tuberculosis is an infection caused by Mycobacterium tuberculosis, which affects tissues and organs outside the pulmonary parenchyma. It represents between 20% and 25% of all cases of TB and the subsequent dissemination of the infection [3]. It affects various organs, such as the lungs, GI tract, skin, bones, and joints [1]. Herein, we present a case of rapidly progressive PV with coexisting extrapulmonary TB and myelosuppression refractory to conventional management treated successfully with rituximab.
CASE REPORT
A 47-year-old female presented with erosions and crusted lesions on the body and oral mucosa. The diagnosis of PV was histologically confirmed. She was treated with prednisone gradually increased to 120 mg/day for one month. After a brief period of improvement new lesions appeared; we, then, combined prednisone with azathioprine. The patient developed a psoas abscess secondary to tuberculosis and was started on extrapulmonary tuberculosis treatment. She also developed steroid-induced idiosyncratic avascular necrosis of the right femur. Hence, steroids were tapered and the dose of azathioprine was increased to 100 mg/day. Again, there was an improvement for a brief period but the disease regressed rapidly, with severe worsening of PV after four weeks (Fig. 1). This time, the patient presented with myelosuppression and a relapse of PV. At this point, we stopped azathioprine and myelosuppression was corrected by three packed cell transfusions at frequent intervals. After the correction of blood counts, 1000 mg of rituximab (RTX) infusion at two-week intervals was initiated, maintaining oral prednisone at 60 mg/day. There was a gradual improvement of the lesions on the trunk in two weeks (Fig. 2). Steroids were gradually tapered and discharged after two months. The patient has been in remission for the last one year after the first cycle of RTX.
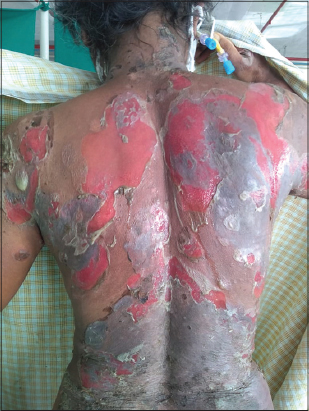 |
Figure 1: Extensive erosions with oozing seen on the back before the infusion of rituximab. |
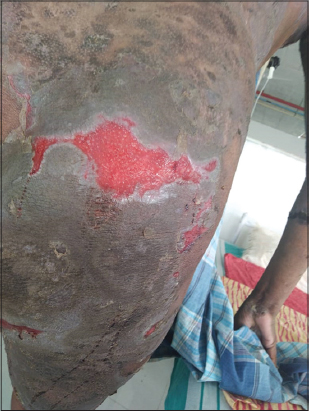 |
Figure 2: Epithelization of the erosions seen two weeks after the infusion of rituximab. |
DISCUSSION
Every patient with PV poses a unique challenge in terms of disease control and adverse events. Diabetes, hypertension, infections, myelosuppression, and other associated complications restrict treatment options. The patient’s quality of life will be compromised with recurrent or recalcitrant disease [4].
The main objective in the management of PV is rapid control of the progression of the disease, healing the lesions, and minimizing the associated morbidity. Subsequently, the aim is to achieve long-term remission and avoid adverse effects associated with prolonged use of immunosuppressants. The real challenge in our case was that the patient had severe PV with extra pulmonary TB and myelosuppression secondary to steroids and azathioprine (AZA). Due to long-term steroid-induced complications such as hypertension, diabetes, and osteoporosis, AZA was started. However, on adding AZA, the patient developed myelosuppression, which was managed by stopping AZA and packed cells transfusions. Extrapulmonary tuberculosis treatment was started for the psoas abscess. The above complications are expected in long-term use (after more than four months) of the immunosuppressant.
Prolonged and high-dose administration of steroids is often needed to control certain autoimmune diseases. Immunosuppression masks signs and symptoms of tuberculosis leading to a delay in diagnosis but also predisposes to more severe variants of tuberculosis. Previous studies have concluded that systemic steroid therapy in the long term causes a significant increase in the incidence of tuberculosis [5]. Numerous mechanisms exist: Steroids through their immunosuppressive and anti-inflammatory effects impair antibody formation and cell-mediated immunity. These effects are most evident if steroid doses exceed 0.03 mg/kg/day of prednisolone or an equivalent. At doses higher than 1 mg/kg/day, a marked increase in susceptibility to a wide variety of infections is experienced after several weeks. Continuous therapy produces longer and more profound immunosuppressive effects when compared with intermittent steroid therapy [5]. In a study conducted on patients with dermatomyositis, taking steroids and azathioprine increased the risk of developing TB [6].
AZA is a cytotoxic drug used in autoimmune diseases, which antagonizes purine metabolism and inhibits the synthesis of DNA, RNA, and proteins. The efficacy of AZA as a corticosteroid-sparing agent in PV is a well-documented and the most often prescribed immunosuppressant. The recommended dose in PV is 1–3 mg/kg/day orally in two separate doses. Its major side effects are pancytopenia, hepatotoxicity, an increased risk of infections, and neoplasms [7].
Rituximab (RTX) is indicated mainly for patients with PV refractory to at least two therapeutic modalities [8]. It is a murine/human chimeric monoclonal antibody against CD20 that induces the depletion of B-cells in vivo. RTX binds human complement, affecting complement-dependent cell lysis, and antibody-dependent cellular cytotoxicity disrupts the signaling pathway and triggers apoptosis [9]. There are two officially approved regimes. The lymphoma regime involves 375 mg/m2 of RTX IV infusion weekly once for 4–8 consecutive weeks. In the rheumatoid arthritis regime, two IV infusions of 1000 mg of RTX are given two weeks apart [9].
In June 2018, the U.S. FDA approved RTX for PV [7]. Schmidt et al. reported a complete remission in 77% and a partial remission in 21% of patients [8]. Serious adverse reactions include angina, adverse drug reactions, intestinal obstruction, lymphocytopenia, anemia, recurrent hepatitis B, progressive multifocal leukoencephalopathy are rare with RTX. The most common adverse effects are fever, chills, bronchospasm, pruritus, hypotension, often related to the rate of infusion and immune hypersensitivity [10,11]. We minimized infusion reactions with prior administration of analgesics, antihistamines, and corticosteroids.
In our case, the patient developed tuberculosis after being treated with prednisolone and myelosuppression with azathioprine for more than a year, and was later was successfully treated with RTX. Such patients should be screened for pulmonary tuberculosis and myelosuppression before and at three-month intervals after the commencement of immunosuppressants and provided with appropriate treatment if needed. There is limited literature on RTX infused in PV with coexisting extrapulmonary TB.
CONCLUSION
Systemic steroids and immunosuppressants used in combination or rotation are the mainstay of management in PV. However, the adverse effects from long-term therapy contribute to morbidity and mortality. In our refractory and clinical rapidly progressive case, RTX led to a longer remission with a faster clinical improvement. RTX is an emerging therapy for numerous autoimmune diseases, especially for recalcitrant and rapidly progressive PV. Further studies are required to determine the dose needed to achieve a long-term clinical remission and cost-effective management.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Talat H, Mirza R, Sajid M, Mansoor M, Wahid Z, Majeed S. Pemphigus vulgaris;Frequency of pulmonary tuberculosis in patients on immunosuppressive drugs. Professional Med J. 2017;24:1081-3.
2. Raviglione MC. Tuberculosis. In:Jameson JL, Fauci AS, Kasper DL et al. Harrison’s Principles of Internal Medicine, 20th ed. New York:McGraw-Hill;2018. P.1236-58.
3. Ramírez-Lapausa M, Menéndez-Saldaña A, Noguerado-Asensio A. Extrapulmonary tuberculosis:An overview. Rev Esp Sanid Penit. 2015;17:3-11.
4. Gregoriou S, Efthymiou O, Stefanaki C, Rigopoulos D. Management of pemphigus vulgaris:Challenges and solutions. Clin Cosmet Investig Dermatol. 2015;8:521-7.
5. Butt G, Asad F, Khurshid K, Rani Z, Pal SS. Frequency of pulmonary tuberculosis in patients with skin diseases requiring high dose long-term systemic steroid therapy. J Pak Assoc Dermatol. 2013;23:126-32.
6. Niyaz A, Viswanath BK. Atypical presentation of pemphigus vulgaris –A rare case report. Our Dermatol Online. 2019;10:88-90.
7. Porro AM, Hans Filho G, Santi CG. Consensus on the treatment of autoimmune bullous dermatoses:pemphigus vulgaris and pemphigus foliaceus –Brazilian Society of Dermatology. An Bras Dermatol. 2019;94:S20-32.
8. Biot SDRN, Franco JPA, Lima RB, Pereira HNC, Marques LPJ, Martins CJ. Refractory pemphigus vulgaris treated with rituximab and mycophenolate mofetil. An Bras Dermatol. 2014;89:980-4.
9. Bomm L, Fracaroli TS, Sodre JL, Bressan A, Gripp AC. Off-label uses of rituximab in dermatology:Pemphigus. An Bras Dermatol. 2013;88:676-8.
10. Bay Bay H, Senhaji G, Elloudi S, Douhi Z, Mernissi FZ. Efficacy of Rituximab in the treatment of pemphigus:Experience of Moroccan population. Our Dermatol Online. 2019;10:338-40.
11. Diallo M, Diatta BA, Diop A, Ndiaye M, Ndiaye MT, Seck B, et al. [Epidemiological and clinical aspects of pemphigus in Senegal]. Our Dermatol Online. 2017;8(Suppl. 1):5-9.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-0240-0433 http://orcid.org/0000-0002-0240-0433 |



Comments are closed.