Efficacy and safety of dupilumab in adult moderate-to-severe atopic dermatitis: An update narrative literature review
Magdalini Kreouzi1, Nikolaos Theodorakis2, Ekatherine Prokopiou1, Elena Thomaidou 1
1
1University of Nicosia Medical School, 21 Ilia Papakyriakou, 2414 Engomi, P.O. Box 24005, CY-1700, Nicosia, Cyprus, 2School of Medicine, National and Kapodistrian University of Athens, 75 Mikras Asias, 11527 Athens, Greece
Corresponding author: Elena Thomaidou, MD
How to cite this article: Kreouzi M, Theodorakis N, Prokopiou E, Thomaidou E. Efficacy and safety of dupilumab in adult moderate-to-severe atopic dermatitis: An update narrative literature review. Our Dermatol Online. 2022;13(1):6-15.
Submission: 30.08.2021; Acceptance: 19.11.2021
DOI: 10.7241/ourd.20221.2
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Adult atopic dermatitis (AD) is defined as a continuum of childhood AD or the development of the disease in adulthood, accounting for 7.7–59.7% of adult AD cases varying in severity and manifestations. The symptomatology of moderate-to-severe adult AD may significantly impact the overall health and quality of life of the patient. The “classic” topical treatments used in mild-to-moderate cases, such as emollients and topical corticosteroids, are usually not adequate to control the symptoms of most of the patients with moderate-to-severe disease. For many years these patients were managed with systemic corticosteroids and immunomodulators, leading to substantial side effects with questionable efficacy. The introduction of dupilumab, the first biologic agent approved by the Food and Drug Administration for use in adult moderate-to-severe AD, has commenced a new era in the management of AD. This narrative literature review addresses the question of how patients with moderate-to-severe AD may achieve a recession or improvement in the overall progression of the disease with the use of dupilumab in both an efficient and safe way.
Material and Methods: A search in the PubMed, Embase, and Cochrane databases was conducted using the following combination of MeSH terms: “dupilumab” AND “atopic” (“dermatitis” OR “eczema”). The searches were limited to RCTs written in the English language published before January 25, 2021. The literature used included phase II and III RCTs examining the efficacy and/or safety of dupilumab compared to placebo or other treatments in adults with moderate-to-severe AD. Moderate-to-severe AD was defined by an IGA score of 3 (moderate) or 4 (severe) and EASI 16 or higher at screening and baseline. Additionally, we searched the website clinicaltrials.gov for any unpublished or ongoing RCTs. The search was done independently by two authors in all databases and followed by the exclusion of duplicates.
Results: Upon reviewing all randomized controlled trials, dupilumab was found to be an effective and safe option for managing adult moderate-to-severe AD with long-term therapeutic effects.
Conclusion: The best results for maintaining long-term disease recession were achieved with the combination of dupilumab and topical corticosteroids.
Keywords: Atopic dermatitis; Biologics; Dupilumab; Efficacy; Safety
INTRODUCTION
Atopic dermatitis (AD) or atopic eczema is a highly prevalent chronic inflammatory skin disorder affecting all ages [1]. In recent years, AD prevalence has increased among several ethnic groups; the highest prevalence of AD between the ages of 13–14 was found in Bolivia and Brazil, with a rate of 21.1%, while other highly prevalent countries include Africa, Oceania, and Northern Europe [2]. The prevalence of the disease in childhood is up to 20% and up to 10% in adults, affecting 14–24% of the general population [3]. Childhood AD may progress into adult AD in about 10–30% of patients [4]. Adult AD is defined as either a continuum of childhood-onset AD or the development of the disease in adulthood; the latter is called adult-onset AD, accounting for 7.7–59.7% of adult AD cases [5]. For the scope of this narrative literature review (NLR), adults will be outlined exclusively.
The term atopy is defined as a predisposition to immunoglobin E (IgE) release after exposure to specific antigens or allergens [6]. AD is often associated with other atopic diseases, such as allergic rhinitis and rhino-conjunctivitis, allergic bronchial asthma, and food allergy, which may be present in the past medical or family history of the patient, a phenomenon known as atopic march [7]. Despite its name, the pathophysiology of the disease is not a typical type 1 hypersensitivity reaction; it includes complex mechanisms. The pathogenesis includes two basic components: a compromised keratin barrier and immune-mediated inflammation driven mainly by a T-helper 2 (Th2) response [8,9]. The former may be a result of various mechanisms (e.g., filaggrin mutations) and leads to epidermal dehydration and increased penetration of various antigens, including microorganisms and allergens [9]. The latter results in increased production of various cytokines, including interleukins IL-4, IL-5, and IL-13. IL-5 induces the activation of eosinophils, which plays a role in the inflammation seen in AD. IL-4 and IL-13 bind to IL-4a, producing various effects, including class switching and the production of IgE by the B-lymphocytes, the differentiation of CD4+ T-lymphocytes into Th2 cells, epidermal dysfunction, itch, and predisposition to skin infections [8]. Dupilumab, the biologic to be outlined in this review, targets the receptor IL-4Rα, therefore blocking the IL-4 and IL-13 signaling pathways. In recent years, various other immunological mechanisms, such as Th1, Th17, and Th22 responses, have been implicated in the pathogenesis of AD [8].
AD is characterized by a pruritic cutaneous rash with specific patterns of involvement: facial, neck, and extensor surfaces, sparing areas such as the groin and axillary region [10]. The diagnosis of AD is mainly clinical, based on specific criteria, including the characteristics of the rash and the past and family history of atopic diseases. Some of these criteria are by Hanifin and Rajka (1980) and the UK Working Party (1994) [11,12]. A skin biopsy may be used only to exclude other conditions, because the histological findings are not pathognomonic for AD, while skin prick testing and allergen-specific IgE testing have been included in the Millennium Criteria (1998) [13]. The severity of AD may be measured with various tools, including the Eczema Area and Severity Index (EASI), Investigation Global Assessment (IGA), Percent of Body Surface Area (BSA), Pruritus Numerical Rating Scale (NRS), Scoring Atopic Dermatitis (SCORAD), Physician Global Assessment (PGA), Atopic Dermatitis Severity Index (ADSI), Global Individual Signs Score (GISS), Six-Area, Six-Sign Atopic Dermatitis (SASSAD), Patient-Oriented Eczema Measure (POEM), Hospital Anxiety Depression Scale (HADS), and Dermatology Life Quality Index (DLQI). These tools help the physician to classify the disease severity according to its symptomatology, to determine the extent of skin involvement, and to gain a better understanding of how the disease affects the quality of life of the individual [14].
The treatment of mild-to-moderate AD is based on the avoidance of specific irritants and allergens and the use of topical emollients, topical corticosteroids (TCS), or topical calcineurin inhibitors. In patients with moderate-to-severe AD, which accounts for approx. 20% of patients with AD, control of the disease is usually inadequate with the above-mentioned treatments and, as a result, phototherapy or systemic medications (e.g., corticosteroids, calcineurin inhibitors, methotrexate, mycophenolate mofetil) are employed [15]. There are numerous adverse effects and problems arising from these treatments, such as increased susceptibility to infection, bone marrow suppression, nephrotoxicity, and hepatotoxicity, hence there has been a need for the development of safe and effective alternatives, which mainly include biological agents and Janus kinase (JAK) inhibitors [16]. Table 1 summarizes all biological agents targeting various aspects of the pathogenesis of AD, which had been on trial until June 29, 2021. In general, most of these RCTs are small phase II trials without published results yet; however, tralokinumab (anti-IL-13) has successfully completed a phase III trial, showing promising results in both efficacy and safety in adult moderate-to-severe AD. Furthermore, there are currently two large phase III studies (RCT and open-label) assessing the efficacy and safety of nemolizumab (anti-IL-31R) in adult moderate-to-severe AD [15,17]. Dupilumab has been proven to be a safe and efficacious therapeutic agent of adult moderate-to-severe AD and was granted approval by the United States Food and Drug Administration (FDA) in 2017 for this indication [18]. This NLR will extensively outline the efficacy and safety of dupilumab from the data obtained from randomized controlled trials (RCTs) with a brief reference to its pharmacological characteristics.
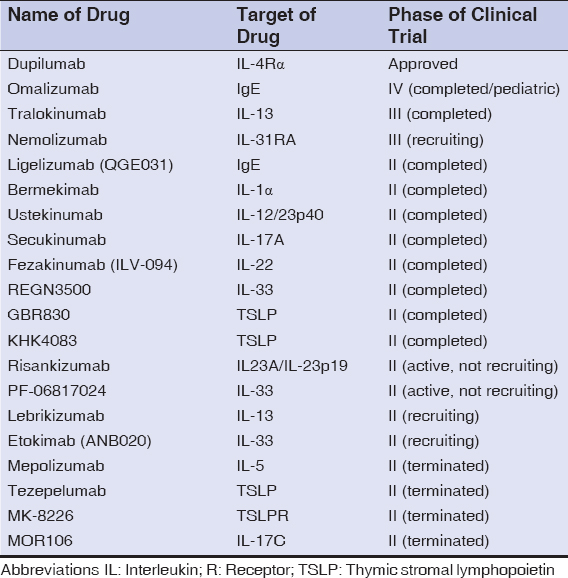 |
Table 1: Summary of all biologics involved in clinical trials for patients with atopic dermatitis. Data obtained from: https://www.clinicaltrials.gov accessed 10 Feb 2021). |
MATERIALS AND METHODS
A search of the PubMed, Embase, and Cochrane databases was conducted using the following combination of MeSH terms: “dupilumab” AND “atopic” (“dermatitis” OR “eczema”). The searches were limited to RCTs written in the English language published before June 29, 2021. The literature used included phase II and III RCTs examining the efficacy and/or safety of dupilumab compared to placebo or other treatments in adults with moderate-to-severe AD. Moderate-to-severe AD was defined by an IGA score of 3 (moderate) or 4 (severe) and EASI 16 or higher at screening and baseline. Additionally, we searched the website clinicaltrials.gov for any unpublished or ongoing RCTs. The search was done independently by two authors in all databases and followed by the exclusion of duplicates.
Molecular Structure and Mechanism of Action
Dupilumab is a fully human IgG4 monoclonal antibody binding to the IL-4Rα subunit, which is shared by both IL-4 and IL-13. IL-4R is characterized by two types: the IL-4Rα/gc complex (type 1) and the IL-4Rα/IL-13Ra complex (type 2). Dupilumab achieves signaling inhibition of IL-4 by blocking both types of receptors and IL-13 signaling by blocking type 2 receptors. As a result, it causes the dual inhibition of the IL-4/IL-13 signaling pathway, producing a reduction in epidermal hyperplasia, modification in the lesional skin appearance, modulation of genes related to epidermal pathology in AD, and inhibition of the release of proinflammatory cytokines, chemokines, and IgE [19–21].
Dosing and Administration
Dupilumab is a biologic characterized by a clear, colorless to slightly yellowish appearance, which is administered subcutaneously via injection. Sites of injection may include the upper arms, thighs, or abdomen, with the exception of the navel and the surrounding 5 cm area. The pharmaceutical company currently supplies the agent in two different strength options: 300 mg/2 mL and 200 mg/1.14 mL, with both options being single-dose injections [19].
Dosing for adults with AD is initially two 300 mg injections (600 mg) administered on different sites each. A maintenance dose is then supplied with a single 300 mg injection every two weeks. If the patient misses a dose of the drug, it is advised that the dose is administered within seven days, which is then followed by the original schedule of the maintenance dose. There is currently no evidence or research being conducted on any adjustments that should be made for patients with renal failure, hepatic failure, or patients in dialysis [20,22–25]. Patients with helminthic infections should not initiate treatment with dupilumab due to the influence of this drug on the immune response during an infection. Thus, patients are advised to resolve the infection and suspend treatment in any reoccurring or new helminthic infections [20,26]. In regard to vaccination during treatment, post-initiation and pre-initiation are discussed in later sections of this NLR.
Pharmacokinetics
The bioavailability of dupilumab after a subcutaneous injection is approx. 64%, while the estimated volume of distribution is 4.8 ± 1.3 L. The maximum serum concentration of dupilumab. Following an initial subcutaneous dose of 600 mg was 70.1 ± 24.1 mcg/mL in one week after the injection. Following administrations of 300 mg weekly or every other week, the steady-state concentration was achieved at week 16. The steady-state concentration ranged from 173 ± 75.9 mg/mL to 193 ± 77.0 mg/mL for 300 mg injected weekly and from 73.3 ± 40.0 mg/mL to 79.9 ± 41.4 mg/mL for 300 mg injected every other week. Dupilumab is metabolized by degradation into smaller peptides and amino acids with the same pathways as endogenous IgG [20,21].
Pharmacodynamics
Early results showed that there is a significant increase in the serum concentrations of IL-4 and IL-13 following dupilumab administration, indicating IL-4Rα blockade [20]. There has also been a recently published study assessing the pharmacodynamics of dupilumab showing statistically significant decreases in total serum IgE, serum thymus, and activation-regulated chemokine (TARC) [21]. TARC is a correlation factor of disease activity as well as the blood eosinophil count.
In two studies, both IgE and TARC serum levels were measured in patients receiving dupilumab therapy with varying results between groups and doses [27,28]. IgE concentrations were found to decline significantly following dupilumab administration, which was observed with increasing dosage. However, a study showed that a dose of 75 mg or 150 mg had no significant effects on the IgE serum decline [27]. In the above studies, the measurement of TARC was also conducted and was found to markedly decrease after dupilumab administration correlated with decreased disease activity compared to placebo. The highest decrease in serum TARC was observed with the use of 300 mg of dupilumab at day eight of treatment, although doses 75–600 mg were all associated with a dose-dependent decline in serum TARC when compared with the placebo [27,28].
Drug Interactions
The concomitant use of dupilumab with live-attenuated vaccines could potentially lead to disseminated infection and thus the administration of live-attenuated vaccines should strictly excide twelve weeks prior to the first administration of dupilumab [19,20]. However, further clinical trials are needed in order to assure this possible interaction between live vaccine use and dupilumab. A 32-week study was conducted to assess the immunization response to non-live vaccines in adults with moderate-to-severe AD treated with dupilumab. The study measured the percentage of participants with a positive response (more than fourfold) to the tetanus toxoid (Tdap) and meningococcal polysaccharide vaccine. Results were highly promising, with the Tdap at 83.3% (compared to the placebo at 83.7%) and for the meningococcal at 86.3% (compared to the placebo at 83.7%) [29].
Furthermore, dupilumab could theoretically alter the formation of cytochrome P450 (CYP) enzymes. Therefore, patients receiving drugs that are CYP substrates, especially those with a narrow therapeutic index or severe side effects, should be monitored for their efficacy (e.g., prothrombin time for warfarin) and/or plasma levels (e.g., cyclosporine) [20]. However, a clinical trial (NCT02647086) conducted to assess drug-to-drug interactions between dupilumab and drugs metabolized by specific CYP enzymes demonstrated that the pharmacokinetics of oral midazolam (CYP3A), omeprazole (CYP2C19), warfarin (CYP2C9), caffeine (CYP1A2), and metoprolol (CYP2D6) were unaffected by dupilumab. Thus, the study concluded that there were no clinically significant and/or relevant effects on the pharmacokinetics of CYP substrate, provided that dupilumab clinically benefited the patients [30].
RESULTS
Efficacy of Dupilumab
The most valuable RCTs evaluating the efficacy of dupilumab were: LIBERTY AD CHRONOS (NCT02260986) (n = 740), LIBERTY AD CAFÉ (NCT02755649) (n = 325), LIBERTY AD SOLO 1 (NCT02277743) (n = 671), LIBERTY AD SOLO 2 (NCT02277769) (n = 708), and LIBERTY AD SOLO-CONTINUE (NCT02395133) (n = 422). All five trials were randomized, double-blinded, placebo-controlled, parallel-group, phase III clinical trials, with SOLO 1 and 2 being replicate trials. The CHRONOS and SOLO 1 and 2 trials assessed the efficacy of dupilumab when compared with the placebo in 52 and 16 weeks, respectively [22,24]. The CAFÉ trial assessed the efficacy of dupilumab when compared with the placebo in 16 weeks in patients who had never received cyclosporin A (CsA) or patients for whom CsA treatment failed [25]. Patients in the CHRONOS, CAFÉ, and SOLO 1 and 2 trials were randomized into three groups: subcutaneous dupilumab 300 mg once weekly (qw group), subcutaneous dupilumab 300 mg every two weeks (q2w group), or placebo once weekly [22–25]. In the CHRONOS and CAFÉ trials, patients from all groups received concomitant TCS (or topical calcineurin inhibitors), compared to the SOLO 1 and 2 trials, which used dupilumab as a monotherapy [22,24,25]. The SOLO-CONTINUE trial assessed the ability of different dupilumab dose regimens to maintain the treatment response achieved in the SOLO 1 and 2 trials compared to the placebo in a time span of 36 weeks. Patients in the SOLO-CONTINUE trial were randomized into four groups: subcutaneous dupilumab 300 mg once/twice weekly (qw/q2w groups), subcutaneous dupilumab 300 mg every four weeks (q4w group), subcutaneous dupilumab 300 mg every eight weeks (q8w group) or placebo once weekly (placebo group) [23]. The (co)primary and secondary outcomes of the five major trials (CHRONOS, SOLO 1 & 2, CAFÉ, and SOLO-CONTINUE) are summarized in Table 2. In addition, table 3 summarises and compares the results for IGA (IGA0/1 plus absolute reduction of two or more from baseline) and EASI-75 (at least a 75% improvement in EASI from baseline) in all trial groups (q2w, qw and placebo) between the CHRONOS, SOLO 1 & 2 and CAFÉ trials. The results are presented in terms of the number and percentage of participants fulfilling the criteria.
For the CHRONOS trial, in the q2w groups, the coprimary outcome for IGA0/1 (IGA0/1 plus the absolute reduction of two or more) was achieved in 41 patients (39%) at week 16 and 32 patients (36%) at week 52, while the coprimary outcome for EASI-75 (at least a 75% improvement from the baseline) was achieved in 73 patients (69%) at week 16 and 58 patients (65%) at week 52. In the qw groups, the coprimary outcome for IGA0/1 occurred in 125 patients (39%) at week 16 and 108 patients (40%) at week 52, while the coprimary outcome for EASI-75 occurred in 204 patients (64%) at week 16 and 173 patients (64%) at week 52. In the placebo groups, the coprimary outcome for IGA0/1 occurred in 39 patients (12%) at week 16 and 33 patients (13%) at week 52, while the coprimary outcome for EASI-75 occurred in 73 patients (23%) at week 16 and 57 patients (22%) at week 52. For all coprimary outcomes, the difference between both the dupilumab groups and the placebo group was statistically significant (p < 0.0001 for all comparisons). Furthermore, both dupilumab groups showed a significant improvement in other secondary outcomes, such as pruritus NRS, HADS, and DLQI, compared to the placebo [24].
For the SOLO 1 and 2 trials, in the q2w groups, the primary outcome (IGA0/1) in 16 weeks was achieved in 85 patients (38%) in SOLO 1 and 84 patients (36%) in SOLO 2, while the most notable secondary outcome (for EASI-75) was achieved in 115 patients (51%) in SOLO 1 and 103 patients (44%) in SOLO 2. In the qw groups, the primary outcome occurred in 83 patients (37%) in SOLO 1 and 87 patients (36%) in SOLO 2, while the EASI-75 outcome occurred in 117 patients (52%) in SOLO 1 and 115 patients (48%) in SOLO 2. In the placebo groups, the primary outcome occurred in 23 patients (10%) in SOLO 1 and 20 patients (8%) in SOLO 2, while the EASI-75 outcome occurred in 33 patients (15%) in SOLO 1 and 28 patients (12%) in SOLO 2. For both the primary and EASI-75 outcomes, the difference between both the dupilumab and placebo groups and in both trials was statistically significant (p < 0.001 for all comparisons). Furthermore, both dupilumab groups showed a significant improvement in other secondary outcomes, such as pruritus NRS, HADS, and DLQI compared to the placebo [22].
In the CAFÉ trial, the primary outcome (EASI-75) was achieved in 32 patients (29.6%) in the placebo group, 67 patients (62.6%) in the q2w group, and 65 patients (59.1%) in the qw group. There were 19 patients (26.4%) with prior use of CsA who achieved an EASI-75 at week 16 in the placebo group, 40 patients (58%) in the q2w group and 39 patients (56.5%) in the qw group. The number of patients who achieved both IGA of 0 or 1 and a reduction of at least 2 from the baseline in 16 weeks was 15 patients (13.9%) in the placebo group, 43 patients (40.2%) in the q2w group, and 43 (39.1%) in the qw group. For all the above-mentioned outcomes, the difference between both the dupilumab groups and the placebo was statistically significant (p < 0.001 for all comparisons). Furthermore, both dupilumab groups showed a significant improvement in other secondary outcomes, such as pruritus NRS, HADS, and DLQI compared to the placebo [25].
In the SOLO-CONTINUE trial, the coprimary outcome for EASI-75 was achieved in 24 patients (30.4%) in the placebo group, 116 patients (71.6%) in the qw/q2w group, 49 patients (58.3%) in the q4w group, and 45 patients (54.9%) in the q8w group. The mean percentage increase of EASI between the baseline and week 36 was 21.67% (placebo group), 0.06% (qw/q2w group), 3.84% (q4w group), and 6.84% (q8w group). For all the above-mentioned outcomes, the difference between both the dupilumab groups and the placebo was statistically significant (p = 0.004 for the EASI-75 in the q8w group, p < 0.001 for all other comparisons). Furthermore, all dupilumab groups and, especially, the qw/q2w group showed statistically significant improvements in other secondary endpoints, such as pruritus NRS, HADS, and DLQI compared to the placebo. [23].
As of June 29, 2021, six other completed phase II (NCT02210780, NCT01979016, NCT01548404, NCT01639040, NCT01859988, NCT03736967) and three phase III (NCT03912259, NCT03720470, NCT03738397) RCTs have examined and outlined the effectiveness of dupilumab in moderate-to-severe AD and have given great insights in the therapeutic approach. Two of these RCTs (NCT03720470 and NCT03738397) include patient groups receiving the JAK inhibitors abrocitinib (PF-04965842) and upadacitinib, respectively. All of the above RCTs showed a significant improvement in the symptomatology, progression of the disease, mental health, sleep disturbances, and health-related quality of life in the dupilumab groups over the placebo, which were measured with several scoring systems [29,31–37].
Currently, there are three ongoing phase III RCTs (NCT04678882, NCT04417894, NCT04345367) assessing the safety and/or efficacy of dupilumab. The NCT04345367 trial aims to compare the effectiveness and safety of the JAK inhibitor abrocitinib over dupilumab [38–40].
Long-term efficacy has been established with the LIBERTY AD OLE, a phase 3, multi-center, open-label extension study with 2733 participants, who received dupilumab 300 mg weekly for 148 weeks. The major outcomes in regards to efficacy at week 148 were favorable, with a mean EASI of 1.4 (-95.4% from the baseline) and a weekly pruritus NRS of 2.2 (-65.4% from the baseline) [41].
Safety of Dupilumab
In the outline of the safety of dupilumab, the most valuable RCTs were the CHRONOS, CAFÉ, SOLO 1, SOLO 2, and SOLO-CONTINUE trials. For these trials, there were four deaths documented; their causes were unrelated to the use of the therapeutic agent. In addition, withdrawal of the participants from the trial due to adverse effects were among all groups; more common in the placebo group, accounting for 1–8%, in comparison to the dupilumab groups q2w accounting for 1–2% and qw 1–3%. Furthermore, the most common of the side effects present and documented throughout all trials was AD exacerbation, which highly impacted the placebo group (14.8-48.8%) and the minority of cases in groups q2w (7.5-32.1%) and qw (8.2-34.5%) [22–25].
A relatively common non-infectious side effect was injection site reactions with a high prevalence among the dupilumab groups, with q2w accounting for 0.9–15%, qw 3.6–19%, and, for the placebo, 0–8%. Another prevalent non-infectious side effect was headaches, which was slightly more prevalent among the dupilumab groups, in comparison to the placebo. Nasopharyngitis and upper respiratory tract infections were also observed, with their prevalence being balanced throughout the three groups. Non-herpetic skin infections were documented with a higher prevalence in the placebo group, in comparison to the q2w and qw dupilumab groups, among which herpetic infections were slightly more prevalent [22–25].
Conjunctivitis with an unspecified cause and allergic conjunctivitis were documented with a higher prevalence in the dupilumab groups (15–20%), in comparison to the placebo (up to 8%). Bacterial and viral conjunctivitis, on the other hand, were generally of a low prevalence between all groups, but the few cases documented were present in the dupilumab groups [22–25].
As far as long-term safety is concerned, there is data available from two open-label studies. The LIBERTY AD OLE study showed a favorable safety profile in a 148-week period, similarly to the safety outcomes of the RCTs, supporting the long-term safety of this biologic [41]. Similar safety outcomes in a 76-week period are being shown by a large, ongoing, multi-center, open-label study evaluating the long-term safety of dupilumab [42].
There is no available data for the effects of dupilumab use during pregnancy. Human IgG is known to cross the placental barrier, yet the effect of dupilumab on the human fetus remains unknown. Animal studies on the administration of homologous anti-IL-4Rα during pregnancy showed no evidence of fetal toxicity or teratogenesis [20]. Currently, there are two ongoing observational studies assessing the effects of dupilumab on pregnancy: one prospective cohort (NCT04173442) and one retrospective cohort (NCT03936335) [43,44]. As with pregnancy, the effects of dupilumab in the newborn during lactation are unknown. Human IgG is present in the milk and the risks to the newborn should be weighted with the benefits to the mother [20].
Overall, the side effects of dupilumab throughout the trials were minor, with some exceptions, and could easily be managed by the participants. Thus, we conclude that the safety profile of the drug is supportive with relatively few side effects and, rarely, cases of severe manifestations [22–25,29,31–37].
Immunogenicity of Dupilumab in RCTs
In the era of biological therapies, immunogenicity is of high importance. Immunogenicity is defined as a humoral or cell-mediated response induced by the introduction of a foreign substance and, in the case of dupilumab, a monoclonal antibody. In the case of biological therapies, the unwanted effects of immunogenicity include an immune response against the antigen leading to the production of anti-drug-antibodies (ADAs), inactivating the therapeutic effects of the treatment [43]. Data provided by the FDA showed that approx. 7% of patients receiving dupilumab (300 mg) for AD develop ADAs after 16 weeks, with 30% of those patients presenting with neutralizing ADAs [20].
The SOLO-CONTINUE trial determined that ADAs occurred in the placebo group at 11%, in the dupilumab group every eight weeks up to 6%, in the dupilumab group every four weeks at 4.3%, in the dupilumab group every two weeks at 1.2%, and the highest prevalence among all was in the dupilumab weekly group [23]. Furthermore, in the CHRONOS trial, there were 7% of patients developing ADAs, among them 2% having a persistent antibody response and 14% had neutralizing antibodies [24].
DISCUSSION
For the past several years, the management of moderate-to-severe AD was restricted to options such as phototherapy, systemic corticosteroids, or systemic immunomodulators [15]. Phototherapy has been proven inconvenient for a large number of patients, as well as to have adverse effects due to UV radiation, such as non-melanoma skin cancer in the long term, limiting its use [46]. Furthermore, patients using systemic corticosteroids and immunomodulators may present with severe and long-term adverse effects, may have a poor clinical response, become refractory, or require large maintenance doses of these systemic medications in order to maintain recession of the disease [1,15,16].
The development of novel alternatives may be the last resort for many individuals who are unresponsive; thus, biological treatments have proven to be of great importance. One of the first biologic treatments, which is proven to help in AD, is dupilumab, and several clinical trials have been conducted to assess its efficacy and safety in the treatment of adult moderate-to-severe AD. The severity of AD is one of the key criteria for selecting the type of treatment [22–25]. Furthermore, the SCORAD index is a good estimator of AD severity, with scores of < 25 being classified as mild, 25–50 as moderate, and > 50 as severe [47].
Dupilumab was the first FDA-approved biologic for the treatment of adult moderate-to-severe AD, providing solutions to the previously mentioned problems. RCTs show that dupilumab is both a safe and effective option. Concerning the efficacy of the drug, RCT results show substantial improvements in objective signs (e.g., the extent of the disease), subjective signs (e.g., pruritus), mental health (i.e., anxiety or depression), and overall quality of life with minor side effects, compared to a placebo [22–25,29,31–37]. The SOLO 1 and 2 trials revealed that monotherapy with dupilumab could provide adequate clinical responses in sixteen weeks with an encouraging safety profile [22]. The SOLO-CONTINUE trial determined that the patients who achieved a positive response in SOLO 1 and 2 should continue receiving dupilumab every week or every other week in order to maintain this response. Dose regimens every four or eight weeks resulted in decreased efficacy, no change in the safety profile, and increased ADA formation [23]. The CAFÉ and CHRONOS trials concluded that the combination of dupilumab and TCS for sixteen weeks is superior to a placebo with TCS in regard to efficacy with minimal side effects [24,25].
The CHRONOS trial assessed safety and efficacy for a total of 52 weeks and the results showed that both dupilumab groups had similar percentages in efficacy outcomes in 52 weeks compared to 16 weeks without more adverse effects [24]. Furthermore, the higher percentages of patients fulfilling the primary outcomes in CAFÉ and CHRONOS compared to SOLO 1 and 2 could be an indicator that topical corticosteroid treatment should be continued long-term in patients receiving dupilumab, as it increases the chances of a positive response [22,24,25].
In all RCTs, the most common side effects linked to dupilumab were mild, including injection-site reactions and headaches. Furthermore, dupilumab administration was not correlated with an increased susceptibility to infections compared to the placebo, which had a higher prevalence of skin infections [22–25,29,31–37]. Conjunctivitis was a relatively common adverse effect with an unknown pathogenesis linked to the administration of dupilumab in patients with AD rather than other diseases, such as asthma, chronic rhinosinusitis, and nasal polyposis [24].
A limitation of the above studies was the absence of statistical comparison between the qw and q2w dupilumab groups, yet clinical data demonstrates that both regimens are safe and effective for treating adult moderate-to-severe AD. However, in the CHRONOS trial, the variability of the primary outcomes was more prevalent in the q2w groups over time. Although limitations may have been present in all trials, the safety profile outline was consistent among all of them, showing no increased risk of infections (serious or opportunistic), both systemic and skin-related [22–25]. Long-term safety and efficacy beyond the 52-week period in the CHRONOS trial were established by the LIBERTY AD OLE open-label study, which showed a favorable safety profile and sustained efficacy in a 148-week period [41]. A second large open-label study showed promising results in regards to safety in a 76-week period [42]. Furthermore, there are two main cohort studies that are currently evaluating the safety of dupilumab during pregnancy without any results published yet [43,44].
Dupilumab administered together with TCS drastically decreased the use of rescue treatments (e.g., systemic corticosteroids), as established by the CHRONOS trial; however, there have been no current studies comparing dupilumab with systemic corticosteroids or immunomodulators [24]. The above is doubtless a gap in evidence, which could be of great importance in both establishing a stronger safety profile regarding dupilumab and minimizing the use of older systemic medications. Finally, a recent study comparing abrocitinib and dupilumab was published, outlining the superiority of the former in decreasing pruritus, which may become an alternative treatment to adult moderate-to-severe AD as well [36].
Currently, there are no other available biological agents for the management of adult moderate-to-severe AD apart from dupilumab. In addition to the promising therapeutic outcomes observed with dupilumab, encouraging clinical results were also demonstrated with the use of some topical (tofacitinib, ruxolitinib, delgocitinib) or oral (abrocitinib, baricitinib, upadacitinib, delgocitinib) JAK inhibitors and the anti-IL-13 biologic tralokinumab [48,49]. Various phase III trials on the JAK inhibitors, as well as a phase III trial on tralokinumab, outlined that these agents are superior to the placebo for various primary (e.g., EASI-75, IGA0/1) and secondary outcomes (e.g., SCORAD, pruritus NRS, DLQI) [48,49].
In conclusion, the administration of dupilumab, the only FDA-approved biologic for AD, is both an effective and safe therapeutic choice for the treatment of adult moderate-to-severe AD, with an even greater impact on disease recession when used concomitantly with TCS.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children:Part I. J Eur Acad Dermatol Venereol. 2018;32:657-82.
2. Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-1258.e23.
3. Perkin MR, Strachan DP, Williams HC, Kennedy CTC, Golding J. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol. 2004;15:221-29.
4. Ellis CN, Mancini AJ, Paller AS, Simpson EL, Eichenfield LF. Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg. 2012;31:S18-S22.
5. Vakharia PP, Silverberg JI. New and emerging therapies for paediatric atopic dermatitis. Lancet Child Adolesc Health. 2019;3:343-53.
6. Bellanti JA, Settipane RA. The atopic disorders and atopy. “strange diseases“now better defined!Allergy Asthma Proc. 2017;38:241-2.
7. Davidson WF, Leung DYM, Beck LA, Berin CM, Boguniewicz M, Busse WW, et al. Report from the National Institute of Allergy and Infectious Diseases workshop on “Atopic dermatitis and the atopic march:Mechanisms and interventions.“J Allergy Clin Immunol. 2019;143:894-913.
8. Guttman-Yassky E, Waldman A, Ahluwalia J, Ong PY, Eichenfield L. Atopic dermatitis:Pathogenesis. Semin Cutan Med Surg. 2017;36:100-3.
9. Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis:Clinical implications. Allergy Asthma Proc. 2019;40:84-92.
10. Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. Guidelines of care for the management of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-51.
11. M. Hanifin, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermato Venereolologica. 1980;60:44-7.
12. Williams JA, Paddock SW, Vorwerk K, Carroll SB. Organization of wing formation and induction of a wing-patterning gene at the dorsal/ventral compartment boundary. Nature. 1994;368:299-305.
13. Bos JD, Leent EJM, Smitt JHS. The millennium criteria for the diagnosis of atopic dermatitis. Exp Dermatol. 1998;7:132-8.
14. Gooderham MJ, Bissonnette R, Grewal P, Lansang P, Papp KA, Hong C. Approach to the assessment and management of adult patients with atopic dermatitis:A consensus document. Section II:Tools for assessing the severity of atopic dermatitis. J Cutan Med Surg. 2018;22(1_suppl):10S16S.
15. Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2019;124:28-35.
16. Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A,et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children:Part II. J Eur Acad Dermatol Venerol. 2018;32:850-78.
17. Wu J, Guttman-Yassky E. Efficacy of biologics in atopic dermatitis. Expert Opin Biol Ther. 2020;20:525-38.
18. Moyle M, Cevikbas F, Harden JL, Guttman-Yassky E. Understanding the immune landscape in atopic dermatitis:The era of biologics and emerging therapeutic approaches. Exp Dermatol. 2019;28:756-68.
19. DUPIXENT®(dupilumab) HCP Website. Updated November, 2020. Accessed April 20, 2021. www.dupixenthcp.com.
20. FDA. HIGHLIGHTS OF PRESCRIBING INFORMATION. Updated March 2017. Accessed April 23, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf.
21. Li Z, Radin A, Li M, Hamilton JD, Kajiwara M, Davis JD, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of dupilumab in healthy adult subjects. Clin Pharmacol Drug Dev. 2020;9:742-55.
22. Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-48.
23. Worm M, Simpson EL, Thaçi D, Bissonnette R, Lacour JP, Beissert S, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis:A randomized clinical trial. JAMA Dermatol. 2020;156:131-43.
24. Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS):A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-303.
25. de Bruin-Weller M, Thaçi D, Smith CH, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable:A placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018;178:1083-101.
26. Tan LD, Schaeffer B, Alismail A. Parasitic (helminthic) infection while on asthma biologic treatment:Not everything is what it seems. J Asthma Allergy. 2019;12:415-20.
27. ClinicalTrials.gov. Safety, Tolerability and Pharmacokinetics of SAR231893 (REGN668) in Healthy Japanese Adult Male Subjects. Updated December 6, 2013. Accessed May 10, 2021. https://clinicaltrials.gov/ct2/show/NCT01537653.
28. ClinicalTrials.gov. Ascending Dose Study of the Safety and Tolerability of REGN668(SAR231893) in Normal Healthy Volunteers. Updared June 17, 2013. Accessed May 10, 2021. https://clinicaltrials.gov/ct2/show/NCT01015027.
29. Blauvelt A, Simpson EL, Tyring SK, Purcell LA, Shumel B, Petro CD, et al. Dupilumab does not affect correlates of vaccine-induced immunity:A randomized, placebo-controlled trial in adults with moderate-to-severe atopic dermatitis. J Am Acad Dermatol. 2019;80:158-167.e1.
30. Davis JD, Bansal A, Hassman D, Akinlade B, Li M, Li Z, et al. Evaluation of potential disease-mediated drug-drug interaction in patients with moderate-to-severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104:1146-54.
31. Guttman-Yassky E, Bissonnette R, Ungar B, Suárez-Fariñas M, Ardeleanu M, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143:155-72.
32. Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-9.
33. Thaçi D, Simpson EL, Beck LA, Bieber T, Blauvelt A, Papp K, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments:A randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40-52.
34. ClinicalTrials.gov. Evaluation of Dupilumab in Chinese Adult Patients With Moderate to Severe Atopic Dermatitis. Updated April 5, 2021. Accessed May 15, 2021. https://clinicaltrials.gov/ct2/show/NCT03912259.
35. ClinicalTrials.gov. Efficacy and Safety of REGN3500 Monotherapy and Combination of REGN3500 Plus Dupilumab in Adult Patients With Moderate-to-Severe Atopic Dermatitis. Updated March 22, 2021. Accessed May 17, 2021. https://clinicaltrials.gov/ct2/show/NCT03736967.
36. Bieber T, Simpson EL, Silverberg JI, Thaçi D, Paul C, Pink AE, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Eng J Med. 2021;384:1101-12.
37. ClinicalTrials.gov. A Phase 3b Multicenter, Randomized, Double-Blind, Double-Dummy, Active Controlled Study Comparing the Safety and Efficacy of Upadacitinib to Dupilumab in Adult Subjects With Moderate to Severe Atopic Dermatitis. Updated Janyary 13, 2021. Accessed May 18, 2021. https://clinicaltrials.gov/ct2/show/NCT03738397.
38. ClinicalTrials.gov. Dupilumab in Japanese Patients With Atopic Dermatitis. Updated January 22, 2021. Accessed May 18, 2021. https://clinicaltrials.gov/ct2/show/NCT04678882.
39. ClinicalTrials.gov. A Study to Evaluate the Efficacy and Safety of Dupilumab in Adult and Adolescent Patients With Moderate-to-Severe Atopic Hand and Foot Dermatitis (Liberty-AD-HAFT). Updated May 20, 2021. Accessed May 24, 2021. https://clinicaltrials.gov/ct2/show/NCT04417894.
40. ClinicalTrials.gov. A PHASE 3B RANDOMIZED, DOUBLE-BLIND, DOUBLE-DUMMY, ACTIVE CONTROLLED MULTI-CENTER STUDY ASSESSING THE EFFICACY AND SAFETY OF ABROCITINIB COMPARED WITH DUPILUMAB IN ADULT PARTICIPANTS ON BACKGROUND TOPICAL THERAPY WITH MODERATE TO SEVERE ATOPIC DERMATITIS. Updated March 24, 2021. Accessed May 26, 2021. https://clinicaltrials.gov/ct2/show/NCT04345367.
41. Beck LA, Thaçi D, Deleuran M, Blauvelt A, Bissonnette R, de Bruin-Weller M, et al. Dupilumab provides favorable safety and sustained efficacy for up to 3 years in an open-label study of adults with moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2020;21:567-77.
42. Deleuran M, Thaçi D, Beck LA, de Bruin-Weller M, Blauvelt A, Forman S, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82:377-88.
43. ClinicalTrials.gov. Post-authorization Safety Study in North America to Monitor Pregnancy and Infant Outcomes Following Administration of Dupilumab During Planned or Unexpected Pregnancy. Updated June 2, 2020. Accessed May 29, 2021. https://clinicaltrials.gov/ct2/show/NCT04173442.
44. ClinicalTrials.gov. Dupilumab and Pregnancy Outcomes:A Retrospective Cohort Study Using Administrative Healthcare Databases (Dupi PODS). Updated October 29, 2020. Accessed May 29, 2021. https://clinicaltrials.gov/ct2/show/NCT03936335.
45. Groot ASD, Scott DW. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28:482-90.
46. Rodenbeck DL, Silverberg JI, Silverberg NB. Phototherapy for atopic dermatitis. Clin Dermatol. 2016;34:607-13.
47. Oranje AP. Practical issues on interpretation of scoring atopic dermatitis:SCORAD Index, objective SCORAD, patient-oriented SCORAD and Three-Item Severity score. Curr Probl Dermatol. 2011;41:149-55.
48. Cartron AM, Nguyen TH, Roh YS, Kwatra MM, Kwatra SG. JAK inhibitors for atopic dermatitis:A promising treatment modality. Clin Exp Dermatol. 2021;46:820-4.
49. Tubau C, Puig L. IL-13 antagonists in the treatment of atopic dermatitis. Immunotherapy. 2021;13:327-44.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0002-4029-2605 http://orcid.org/0000-0002-4029-2605 http://orcid.org/0000-0003-0420-7327 http://orcid.org/0000-0003-0420-7327 http://orcid.org/0000-0002-8466-2065 http://orcid.org/0000-0002-8466-2065 http://orcid.org/0000-0002-8029-0209 http://orcid.org/0000-0002-8029-0209 |




Comments are closed.