A case of generalized morphea complicated by autoimmune hepatitis
Chihiro Himori1,2, Yoshio Kawakami 1,3, Kazuya Kariyama2, Wakako Oda4, Toshiyuki Yamamoto5
1,3, Kazuya Kariyama2, Wakako Oda4, Toshiyuki Yamamoto5
1Department of Dermatology, Okayama City Hospital, Okayama, Japan, 2Department of Liver Disease Center, Okayama City Hospital, Okayama, Japan, 3Department of Dermatology, Kurashiki Medical Center, Kurashiki, Japan, 4Department of Pathology, Okayama City Hospital, Okayama, Japan, 5Department of Dermatology, Fukushima Medical University, Fukushima, Japan
Corresponding author: Dr. Yoshio Kawakami
Submission: 04.07.2020; Acceptance: 10.10.2020
DOI: 10.7241/ourd.20211.31
Cite this article: Himori C, Kawakami Y, Kariyama K, Oda W, Yamamoto T. A case of generalized morphea complicated by autoimmune hepatitis. Our Dermatol Online. 2021;12(1):99-100.
Citation tools:
Copyright information
© Our Dermatology Online 2021. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
Sir,
Morphea, also known as localized scleroderma, is a fibrosing disorder of the skin and underlying tissues. Morphea is differentiated from systemic sclerosis based on the absence of sclerodactyly, Raynaud’s phenomenon, and nail fold capillary changes [1]. It is mainly classified into five groups: plaque, generalized, bullous, linear, and deep [2]. With certain exceptions, it produces no serious systemic complications but is sometimes associated with autoimmune disorders, such as Hashimoto thyroiditis, rheumatoid arthritis, and alopecia areata [3]. Herein, we report a case of generalized morphea (GM) complicated by autoimmune hepatitis (AIH).
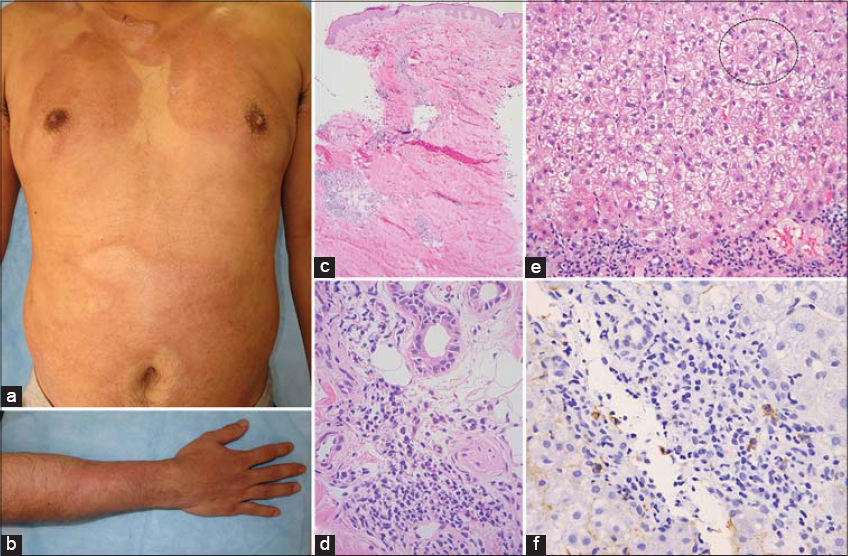
In 2002, a 27-year-old male presented himself with generalized sclerotic skin lesions, which he noticed two months earlier. There were no other systemic symptoms, and the patient denied exposure to organic solvents. A clinical examination revealed multiple well-defined, indurated, ivory-colored plaques on the trunk and extremities (Fig. 1a and 1b). Neither sclerodactyly (Fig. 1b) nor Raynaud’s phenomenon was present. A histopathologic examination of the upper arm revealed swollen collagen bundles (Fig.1c) and inflammatory cell infiltrate composed of lymphocytes and plasma cells around blood vessels and sweat glands (Fig. 1d). A diagnosis of GM was reached and oral prednisolone 40 mg/day was initiated. Following the improvement of the skin lesions, prednisolone was gradually tapered to 5 mg/day. However, the patient failed to attend the scheduled clinical appointment and discontinued the prescribed medication in 2007. In 2013, the patient presented himself with an aggravation of the preexisting skin lesions. Laboratory tests revealed increased liver values (AST at 300 IU/L, ALT at 835 IU/L, GGT at 157 IU/L, and total bilirubin at 2.9 mg/dL). There was an increase in the serum level of IgG at 3,485 mg/dL and IgA at 816 mg/dL. An immunological investigation revealed positive anti-nuclear antibodies (ANA) (1:160, homogeneous), with negative antibodies to smooth muscle, mitochondrial, liver-kidney microsome type 1 (LKM-1), centromere, Scl-70, RNA polymerase III, U1 RNP, dsDNA, and histone. Viral serologies, including the anti-hepatitis A IgM antibody, hepatitis B surface antigen, and anti-hepatitis C virus antibody, were negative. A liver biopsy revealed lymphocellular and plasmocellular infiltrate in portal areas and rosetting of hepatocytes (Fig. 1e and 1f). He denied a history of hepatotoxic medication or alcohol abuse. A diagnosis of AIH was reached and the patient was administered intravenous methylprednisolone 500 mg/day for three days followed by oral prednisolone 40 mg/day. The patient has been in a remission of AIH with no progression of the skin lesions over five years on a maintenance dose of prednisolone 5 mg/day.
AIH is a chronic inflammatory liver disease characterized by elevated serum aminotransferases, elevated IgG, the presence of autoantibodies, and interface hepatitis with plasma cell infiltration in liver biopsies. AIH is often associated with autoimmune disorders such as autoimmune thyroiditis, and ulcerative colitis [4], whereas there have been only two cases of AIH associated with morphea: One case involved GM affecting the extremities and the trunk [5]; the other was located on the buttock and on the right thigh [6]. In both, steroid-based immunosuppressive therapies were initiated [5,6]. A recent review of 245 cases of morphea revealed that GM produces a higher prevalence of concomitant autoimmune disorders and ANA positivity than the other types of morphea, such as plaque or linear [7]. In our case, the development of AIH after discontinuation of prednisolone reinforces the opinion that GM is a systemic disorder and ought to be monitored against the presence of autoimmune disorders and treated with immunosuppressant drugs if indicated.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Fett N, Werth VP. Update on morphea:part I. Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-28.
2. Peterson LS, Nelson AM, Su WP. Classification of morphea (localized scleroderma). Mayo Clin Proc. 1995;60:1068-76.
3. Kreuter A, Wischnewski J, Terras S, Altmeyer P, Stücker M, Gambichler T. Coexistence of lichen sclerosus and morphea:A retrospective analysis of 472 patients with localized scleroderma from a German tertiary referral center.J Am Acad Dermatol. 2012;67:1157-62.
4. Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193-213.
5. Miyagawa F, Asada H. Reactivity to anti-RNA polymerase III antibody and nailfold capillary changes in patient with generalized morphea combined with multiple autoimmune disorders. Eur J Dermatol. 2018;28:112-3.
6. Khalifa M, Ben Jazia E, Hachfi W, Sriha B, Bahri F, Letaief A. [Autoimmune hepatitis and morphea:A rare association]. Gastroenterol Clin Biol. 2006;30:917-8.
7. Leitenberger JJ, Cayce RL, Haley RW, Adams-Huet B, Bergstresser PR, Jacobe HT. Distinct autoimmune syndromes in morphea:A review of 245 adult and pediatric cases. Arch Dermatol. 2009;145:545-50.
Notes
Source of Support: Nil.
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0001-5609-6118 http://orcid.org/0000-0001-5609-6118 http://orcid.org/0000-0002-8390-2573 http://orcid.org/0000-0002-8390-2573 |



Comments are closed.