An investigation of the eff n investigation of the eff ects of acitretin on erectile ects of acitretin on erectile function
Ilteris Oguz Topal 1, Alper Otunctemur2
1, Alper Otunctemur2
1Department of Dermatology, Okmeydani Training and Research Hospital, Istanbul, Turkey, 2Department of Urology, Okmeydani Training and Research Hospital, Istanbul, Turkey
Corresponding author: Dr. Ilteris Oguz Topal
Submission: 21.02.2020; Acceptance: 23.04.2020
DOI: 10.7241/ourd.2020S3.1
Cite this article: Topal IO, Otunctemur A. An investigation of the effects of acitretin on erectile function. Our Dermatol Online. 2020;11(Supp. 3):1-5.
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Acitretin is a second-generation oral retinoid compound used as a treatment option for various dermatological disorders. The effects of the drug on erectile function have not been fully determined yet. We aimed at investigating whether patients taking acitretin develop erectile dysfunction (ED).
Materials and Methods: The study included 40 male patients who presented to the Dermatology Polyclinic of Okmeydan? Training and Research Hospital between October 2014 and April 2016 and started treatment with acitretin. Exclusion criteria included psychogenic and psychiatric disorders, neurological disorders such as multiple sclerosis, diabetes mellitus and endocrine disorders, arteriogenic and venous disorders, alcohol and tobacco use, penile disorders, obesity, a history of drug use, a score of above 10 points in the Beck Depression Inventory (BDI), hyperlipidemia, and an age above 65 years. Having obtained the informed consent, the patients were asked to complete the International Index of Erectile Function (IIEF) questionnaire before and after three months of therapy. The patients were evaluated by calculating their scores and a statistical analysis was performed.
Results: A total of 40 patients were included in the study. A comparison of the IIEF scores before and after three months of therapy revealed that the scores were significantly lower after three months of therapy (P < 0.0001). ED was diagnosed in 30 patients (75%) at the beginning of therapy and in 35 patients (87.5%) after three months of therapy. A comparison of baseline and 3-month IIEF grades revealed that the number of patients in the second group was significantly greater after three months of therapy with the drug (P = 0.001). When the percentage of patients with erectile dysfunction at the baseline and after three months of therapy were compared, no significant change was observed in the percentage of ED at three months (P = 0.11).
Conclusions: Because our study revealed that acitretin may cause ED, we believe that patients should be informed of this potential side effect before initiating treatment.
Key words: Retinoid; Hyperlipidaemia; Acitretin; Erectile dysfunction; Psychiatric
INTRODUCTION
Acitretin is a second-generation oral retinoid used by dermatologists for the treatment of dermatological disorders such as psoriasis, pityriasis rubra pilaris, and lichen planus [1]. Acitretin may cause some common side effects such as dry skin and mucosa, hyperlipidemia, muscle and joint pain, and hepatitis [2].
Erectile dysfunction is defined as the inability to achieve and maintain a penile erection adequate for satisfactory sexual intercourse. Erectile dysfunction may be due to various reasons, including psychogenic and organic factors, endocrine disorders, and drug use [3].
Data on the effect of acitretin on sexual function is limited. The literature provides no studies that would investigate whether acitretin causes erectile dysfunction in humans. Therefore, in this study, we aimed at investigating whether patients taking acitretin develop erectile dysfunction
MATERIALS AND METHODS
The study included 40 male patients who presented to the Dermatology Polyclinic of Okmeydan? Training and Research Hospital between October 2014 and April 2016 and started treatment with acitretin for various dermatological disorders. Exclusion criteria included psychogenic and psychiatric disorders, diabetes mellitus, neurogenic disorders such as multiple sclerosis, endocrine disorders, arteriogenic and venous disorders, alcohol and tobacco use, penile disorders, obesity, a history of drug use, a score of above 10 points in the Beck Depression Inventory, hyperlipidemia, and an age above 65 years.
The study was approved by the ethics committee of Okmeydan? Training and Research Hospital. All participants provided written consent as approved by the local ethics committee.
The patients were asked to complete the International Index of Erectile Function (IIEF) questionnaire, of which the Turkish version was validated by Turunc et al., before the initiation of the treatment [4,5]. The biochemical parameters of cholesterol and triglyceride levels were measured for all patients at the baseline. The IIEF questionnaire was conducted again after three months of therapy and blood lipid values were measured. Patients with hyperlipidemia, as diagnosed on control examinations, were excluded. The patients were asked to complete the Beck Depression Inventory before and after three months of therapy. Patients with a calculated score of above 10 points were excluded [6,7].
The IIEF-5 questionnaire consisted of 5 questions. The patients were given a score between 1 and 5 based on their responses. Patients who scored 6 to 10 points, based on their responses, were included in the group of severe erectile dysfunction (first group); patients who scored 11 to 16 points were included in the group of moderate ED (second group); and patients who scored 17 to 21 points were included in the group of mild to moderate ED (third group). Patients with scores of 22 to 25 were not considered as having ED and were included in the fourth group. Following the calculation of the scores, a statistical analysis was conducted.
STATISTICAL ANALYSIS
Descriptive statistics of the measurements were calculated as means, Standard Deviations (SD), numbers, and percentages for frequencies and provided in tables. Mean scores before and after the drug were compared with a paired samples t-test. The Wilcoxon signed-rank test was used to compare the baseline and the three-month grades. The significance of the change that occurred in ED outcomes at three months was evaluated with the McNemar’s test. In addition, the relationship between the drug dose and the change in score was evaluated with Pearson’s correlation analysis and the independent samples t-test was used to determine if any difference occurred between the drug dose in patients with and without ED. Statistical significance was set at P < 0.05 and the software SPSS, version 18, was used for calculation.
RESULTS
A total of 40 patients aged 23–61 years (median: 41.2 years) were included in the study. These patients were given acitretin doses ranging from 10 to 35 mg.
A comparison of IIEF scores before and after three months of therapy revealed that the scores were significantly lower after three months of therapy (P < 0.0001) (Table 1).
 |
Table 1: A comparison of the IIEF scores before and after three months of therapy |
When IIEF grades were compared before and after three months of therapy, it was observed that the number of patients in the second group was significantly higher after therapy, while the number of patients in the third and fourth groups was significantly lower after therapy (P = 0.001) (Table 2).
 |
Table 2: A comparison of the IIEF grades before and after three months of therapy |
Erectile dysfunction was detected in 30 patients (75%) at the baseline (grade 2 in 12 patients and grade 3 in 18 patients). Erectile dysfunction was diagnosed in 35 patients (87.5%) after three months of therapy (grade 3 in 13 patients and grade 2 in 22 patients).
When the percentage of patients with erectile dysfunction at the baseline and after three months of therapy were compared, no significant change was observed in the percentage of erectile dysfunction at three months (P = 0.11) (Table 3).
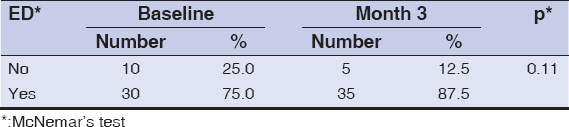 |
Table 3: A comparison of the percentage of patients with erectile dysfunction at the baseline and after three months of therapy |
The relationship between the change observed when the three-month scores were subtracted from baseline scores (difference between the baseline score and the three-month score) and the dose of the drug was investigated and no significant linear relationship was noted (r = 0.151, p = 0.352). This suggests that the change in score was not affected by the change in drug dose. In other words, no correlation could be found indicating that the change in score increases or decreases with the increase in drug dose.
Also, no significant difference was observed in doses taken by patients with and without ED at the baseline (P = 0.362), and the mean dose taken by the patients with ED was significantly higher at three months (P = 0.020). The mean values of drug doses taken by patients with and without ED at the baseline and at three months are provided in Table 4.
 |
Table 4: A comparison of drug doses taken by the patients with and without erectile dysfunction |
DISCUSSION
Retinoids are metabolites and synthetic analogs of vitamin A that play a very important role in dermatological treatments. Acitretin is a retinoid derivative and has been used in the treatment of dermatological disorders since 1988 [2]. Acitretin is a known teratogen that falls into group X, according to the FDA pregnancy classification, and is, thus, contraindicated during pregnancy [1]. Pregnancy is discouraged for three years after discontinuation of the drug in female patients. Such a limitation does not apply to male patients [8]. A study revealed that the amount of acitretin in the seminal fluid of male patients taking acitretin was 1/200,000 of the oral dose. Therefore, acitretin is considered to pose a minimal risk to the fetus while the male patient is taking the drug [9].
There have been some studies in the literature that investigated the effects of acitretin on spermatogenesis. A study of male lizards treated with all-trans retinoic acid revealed that retinoic acid severely depleted the seminiferous epithelium and, therefore, had a significant effect on spermatogenesis [10]. Lauharanta et al. performed the first study evaluating the relationship between acitretin and spermatogenesis. In this study, synthetic retinoids—including etretinate, acitretin, isotretinoin, and retinoic acid—were reported to show dose-dependent inhibition of fructolysis that evaluates sperm motility in ejaculated human spermatozoa in vitro [11]. However, other studies showed no effect of the drug on spermatogenesis. In our country, Sengor et al. conducted a study on rats and determined that the standard and high doses of acitretin did not affect spermatogenesis in rats [12].
There have been some case reports indicating that retinoids may cause sexual dysfunction. Rossi et al. reported erectile dysfunction in a 39-year-old patient with psoriasis after initiation of acitretin therapy. On a follow-up visit, the patient reported an inability to reach and maintain a penile erection sufficient enough to perform a sexual act, beginning from about 45 days after the initiation of therapy [13]. The literature provides two more case reports on the development of impotence associated with the use of etretinate. The development of impotence was observed after the readministration of the drug without the symptoms of depression in one of these cases [14,15].
Although the effects of retinoids on sexual function are not well-known, their effects on the male reproductive system have been investigated in some animal studies [16]. In a study conducted by Csaba et al., rats were divided into three groups: group one was given a single dose of retinol during the neonatal period on day 1 of birth; group two was given retinoic acid subcutaneously on days 1, 3, and 5; and group three was the control group. The sexual activity of the animals was assessed at month 4. The number of inactive males in the group given retinol was observed to be two times higher than in the control group (P < 0.02) [17].
The sexual activity of the animals in the group given retinoic acid was observed to be similar to that of the animals in the control group. However, it was noted that the number of single ejaculations was higher in the treatment group than in the control group (P < 0.02), while the number of multiple ejaculations was higher in the control group (P > 0.05), with a delay in time to the first ejaculation in the treatment group (P < 0.01). The authors suggested that neonatal retinoid exposure may have affected sexual parameters because of the binding of nuclear steroid receptors. They reported that there might have been a deficiency in the selectivity of the steroid receptor capacity during early development and these receptors might have caused the abnormal imprinting by binding retinoids [17].
Previous experimental studies have also shown that the perinatal imprinting with retinol in adults causes an increase in the concentration of glucocorticoid receptors in the thymus and the affinity of uterine estrogen receptors [18].
In our study, a comparison of IIEF scores of patients before and after three months of therapy showed a significant reduction in scores after three months of therapy (P < 0.0001). Likewise, a comparison of baseline IIEF grades and three-month IIEF grades revealed a significant increase in the number of patients in the second group at three months (P = 0.001). However, when the percentage of patients with erectile dysfunction at the baseline and after three months of therapy were compared, a clinically significant increase of 12.5% was observed in the percentage of erectile dysfunction at three months, but without a statistical significance (P = 0.11). We believe that this might have been associated with the small number of patients studied.
The mechanism by which acitretin causes erectile dysfunction is unclear. As is known, retinoid receptors are members of a large receptor family that includes glucocorticoids, thyroid hormone, and the vitamin D3 receptor. There are two different nuclear receptor families: RAR (retinoic acid receptors) and RXR (retinoid X receptors). Retinoid X receptors may form a homodimer with another RXR receptor or a heterodimer with other nuclear receptors, such as thyroid hormone or the steroid receptor [19]. Therefore, the possible mechanism seems to be the inhibition of the activity of testosterone by retinoids by binding to the same site at the receptor molecule.
Testosterone plays an important role in the continuation of normal sexual function. Some animal studies found that a deficiency in testosterone may cause ED [20]. Thus, hormone levels may be questioned in patients who have developed retinoid-associated ED.
Some studies investigated the effects of retinoids on hormone levels as well as their androgenic effects but the results were contradictory. A study conducted by Karadag et al. showed that isotretinoin may lead to a slight reduction in pituitary hormone and testosterone levels [21]. Likewise, Rademaker et al. also observed a marked reduction in serum testosterone levels as a result of isotretinoin therapy [22].
However, there have also been studies in the literature that found no isotretinoin-related changes in hormone levels. Torok. et al. observed no significant difference in total testosterone, LH, or FSH with isotretinoin therapy [23]. Similarly, Marynick et al. found no statistically significant changes in serum DHEAS, total testosterone, LH, or FSH with isotretinoin therapy [24].
Gokalp et al. found no statistical difference between total testosterone levels before and after six months of isotretinoin therapy. It was concluded, hence, that isotretinoin does not exert its antiandrogenic effects through total testosterone [25].
Given the controversial results of these studies, we believe that it would be wrong to establish a direct relationship between ED and hormone levels. Rather, we propose that it would be more reasonable to establish such a relationship with the mechanism through binding to steroid receptors.
Nevertheless, our study had its limitations. One was the limited number of patients and the lack of control groups. This was because the patients were unwilling to take part in such a study, and it was difficult to convince them to participate, given the Muslim country that they lived in and the consequent sociocultural differences. In addition, a large number of exclusion criteria had to be selected, as many factors may cause erectile dysfunction.
Another limitation was our inability to evaluate hormone levels. In conclusion, our study showed that acitretin, a retinoid derivative, has the potential to cause ED. We believe, therefore, that patients should be informed of this side effect before initiating treatment.
Statement of Human and Animal Rights
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 2008 revision of the Declaration of Helsinki of 1975.
Statement of Informed Consent
Informed consent for participation in this study was obtained from all patients.
REFERENCES
1. Heath MS, Sahni DR, Curry ZA, Feldman SR. Pharmacokinetics of tazarotene and acitretin in psoriasis. Expert Opin Drug Metab Toxicol. 2018;14:919-27.
2. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis:A review. JAMA. 2020;323:1945-60.
3. Muneer A, Kalsi J, Nazareth I, Arya M. Erectile dysfunction. BMJ. 2014;348:g129.
4. Neijenhuijs KI, Holtmaat K, Aaronson NK, Holzner B, Terwee CB, Cuijpers P, et al. The International Index of Erectile Function (IIEF)-A systematic review of measurement properties. J Sex Med. 2019;16:1078-109.
5. Mert KU, Dural M, Mert GÖ, Iskenderov K, Özen A. Effects of heart rate reduction with ivabradine on the international ?ndex of erectile function (IIEF-5) in patients with heart failure. Aging Male. 2018;21:93-8.
6. von Glischinski M, von Brachel R, Hirschfeld G. How depressed is “depressed“?A systematic review and diagnostic meta-analysis of optimal cut points for the Beck Depression Inventory revised (BDI-II). Qual Life Res. 2019;28:1111-8.
7. Y?lmaz E, Kavak F. The Effect of stigma on depression levels of Turkish women with infertility. perspect Psychiatr Care. 2019;55:378-82.
8. Millsop JW, Heller MM, Eliason MJ, Murase JE. Dermatological medication effects on male fertility. Dermatol Ther. 2013;26:337-46.
9. Rademaker M, Agnew K, Andrews M, Armour K, Baker C, Foley P, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100.
10. Effect J Hallak, TA Teixeira, GL de Souza. Effect of exogenous medications and anabolic steroids on male reproductive and sexual health. In:Parekattil SJ, Esteves SC, Agarwal A, editors. Male Infertility. 2nd edition, Switzerland:Springer, 2020:455-68.
11. Zakhem GA, Motosko CC, Mu EW, Ho RS. Infertility and teratogenicity after paternal exposure to systemic dermatologic medications:A systematic review. J Am Acad Dermatol. 2019;80:957-69.
12. Koh YP, Tian EA, Oon HH. New changes in pregnancy and lactation labelling:Review of dermatologic drugs. Int J Womens Dermatol. 2019;5:216-26.
13. Molina-Leyva A, Salvador- Rodriguez L, Martinez-Lopez A, Ruiz-Carrascosa JC, Arias-Santiago S. Between psoriasis and sexual and erectile dysfunction in epidemiologic studies:A systematic review. JAMA Dermatol. 2019;155:98-106.
14. Zakhem GA, Goldberg JE, Motosko CC, Cohen BE, Ho RS. Sexual dysfunction in men taking systemic dermatologic medication:A systematic review. J Am Acad Dermatol. 2019;81:163-72.
15. Zhao S,Wang J, Xie Q, Liu Y, Luo L, Zhu Z, et al. High prevalence of erectile dysfunction in men with psoriasis:Evidence from a systematic review and Mmta-analysis. Int J Impot Res. 2019;31:74-84.
16. Sarkar R, Chugh S, Garg VK. Acitretin in dermatology. Indian J Dermatol Venereol Leprol. 2013;79:759-71.
17. Csaba G. Dangerous faulty perinatal imprinting by medication:Review and hypothesis. Clin Obstet Gynecol Reprod Med. 2019;5:1-4.
18. Csaba G. Lifelong impact of perinatal endocrine disruptor exoosures (faulty hormonal imprinting). Int J Plant Animal Environment Scien. 2019;9:94-102.
19. Karada?S, Topaloglu Demir F. Systemic Retinoids. Turkiye Klinikleri J Dermatol-Special Topics. 2014;7:54-70.
20. Retzler K. Erectile dysfunction:A review of comprehensive Treatment options for optimal outcome. J Restor Med. 2019;e20190104.
21. Karadag AS, Takci Z, Ertugrul DT, Bilgili SG, Balahoroglu R, Takir M. The effect of different doses of ?sotretinoin on pituitary hormones. Dermatology. 2015;230:354-9.
22. Abdelmaksoud A, Lotti T, Anadolu R, Goldust M, Ayhan E, Dave DD, et al. Low dose of isotretinoin:A comprehensive review. Dermatol Ther. 2020;33:e13251.
23. Kumar P, Das A, Ranjan Lal N, Jain S, Ghosh A. Safety of important dermatological drugs (retinoids, immune suppressants, anti androgens and thalidomide) in reproductively active males with respect to pregnancy outcome:A brief review of literature. Indian J Dermatol Venereol Leprol. 2018;84:539-46.
24. Zhou JN, Fang H. Transcriptional regulation of corticotropin-releasing hormone gene in stress response. IBRO Rep. 2018;5:137-46.
25. Gökalp H, Aksakal AB. Comparison of the efficacy on serum androgenic hormone levels between isotretinoin, cyproterone acetate/ethynil estradiol and combination therapies in females with acne vulgaris. Turkderm. 2012;46:206-9.
Notes
Source of Support: Nil.
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0001-8735-9806 http://orcid.org/0000-0001-8735-9806 |



Comments are closed.