Severe hypoglycemia and fainting caused by the use of levofloxacin in a nondiabetic patient
Kousar Jahani Amiri1, Ramina Mofarrah 2, Navid Heydar Zadeh3, Melody Omrani Nava4, Alborz Ahmadi1, Melika Ashoorinezhad1, Ramin Mofarrah5
2, Navid Heydar Zadeh3, Melody Omrani Nava4, Alborz Ahmadi1, Melika Ashoorinezhad1, Ramin Mofarrah5
1Student Research Committee, Sari Branch, Islamic Azad University of Medical Sciences, Sari, Iran; 2Medical student, Student Research Committee, Shiraz Branch, University of Medical Sciences, Shiraz, Iran; 3Faculty of Medicine, Sharood Branch, Islamic Azad University of Medical Sciences, Sari, Iran; 4Department of Infectiouse and Tropical Disease, Faculty of Medicine, Sari Branch, Islamic Azad University of Medical Sciences, Sari, Iran; 5Department of Dermatology, Faculty of Medicine, Sari Branch, Islamic Azad University of Medical Sciences, Sari, Iran
Corresponding author: Dr. Ramin Mofarrah
Submission: 10.01.2020; Acceptance: 12.03.2020
DOI: 10.7241/ourd.20203.9
Cite this article: Amiri KJ, Mofarrah R, Zadeh NH, Nava MO, Ahmadi A, Ashoorinezhad M, Mofarrah R. Severe hypoglycemia and fainting caused by the use of levofl oxacin in a nondiabetic patient. Our Dermatol Online. 2020;11(3):264-266.
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Fluoroquinolone-type antimicrobials can cause hypo- or hyperglycemia in certain patients. We reported the case of a young male with no history of any condition and use of medication present with cellulitis. He developed hypoglycemic episodes with levofloxacin. Levofloxacin can cause new onset hypoglycemia and fainting in non-diabetic patients. This study suggests that levofloxacin can cause hypoglycemia even in nondiabetic and previously healthy patients. The seriousness of hypoglycemia and the few reported deaths also underscore the importance of early detection and appropriate management of patients with hypoglycemia.
Key words: Fluoroquinolone, Hypoglycemia, Levofloxacin
INTRODUCTION
Fluoroquinolones have been widely used for the treatment of community- and hospital-acquired infections. These drugs are known to cause glycemic disturbances mainly in patients with diabetes taking either oral hypoglycemic agents or insulin [1,2]. Gatifloxacin was banned in India on March 18th, 2011 because it poses a 17 times higher risk of developing serious hyperglycemia. Although uncommon, hypoglycemia has also been reported with fluoroquinolones. Hypoglycemia typically occurs within the first 3 days of fluoroquinolone therapy and has also been reported after the first dose of either intravenous or oral administration [1]. Most of the patients who developed hypoglycemia following fluoroquinolone use had risk factors, such as old age, diabetes, renal insufficiency, and a concomitant use of hypoglycemic drugs, especially sulfonylureas [2]. Fluoroquinolones have caused at least 67 cases of life-threatening hypoglycemic coma, including 13 deaths and permanent or disabling injuries, according to an internal safety review by the food and drug administration. Most cases (44) were associated with levofloxacin [3] and also new neuropsychiatric side-effects related to fluoroquinolones, including disturbances in attention, memory impairment, and delirium. Considering these findings, the agency will strengthen warning labels on all fluoroquinolones, especially for older people [3]. We reported a case of hypoglycemia associated with levofloxacin administration in a patient without diabetes and history of any medication therapy.
CASE REPORT
A 30-year-old male without any history of disease or use of medication came to our office with pain and swelling in his left leg (Fig. 1). MRI reported a collection of soft tissue in his left leg. He was treated empirically with a levofloxacin tablet of 500 mg once a day due to the diagnosis of cellulitis. After the first dose of levofloxacin, he experienced weakness and dizziness, and after the second dose of levofloxacin, he unexpectedly fainted and was quickly taken to the emergency room. The blood sugar level was detected at 45 mg/dl. He received one vial of dextrose 50% in addition to the standard intravenous dose of saline serum 500 cc. Levofloxacin was discontinued after 2 days, after it was suspected to have caused hypoglycemia. After the discontinuation of levofloxacin, the symptoms of hypoglycemia did not reoccur.
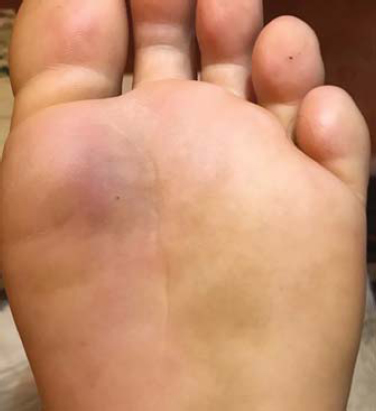 |
Figure 1: Cellulitis of left foot (plantar). |
DISSCUSION
Hypoglycemia is a rare but known, potentially adverse effect of fluoroquinolone therapy [4]. Published reports are available for hypoglycemia in connection with ciprofloxacin, levofloxacin, gatifloxacin, moxifloxacin, and clinafloxacin [1]. Several published case reports have especially implicated levofloxacin as the causative agent of hypoglycemia, with some resulting in fatal outcomes [4]. Due to hypoglycemia having the potential to lead to serious morbidity and mortality, it is important for clinicians to recognize risk factors associated with this adverse event and increase monitoring or choose an alternative therapy when appropriate [5]. Several risk factors may predispose patients to hypoglycemia while being treated with fluoroquinolones, including diabetes, a concomitant use of sulfonylureas or insulin, renal insufficiency, and old age, which is generally defined as patients aged more than 65 [4]. However, our patients have no risk factors. To our knowledge, levofloxacin poses a higher risk of hypoglycemia compared to other fluoroquinolones. The mechanism that causes fluoroquinolones to induce hypoglycemia has not yet been fully elucidated. However, in vitro and animal model studies have provided evidence on proposed pharmacodynamic pathways that modulate insulin secretion. These studies, in addition to the known pharmacokinetic profile of levofloxacin, provide potential explanations for this clinical scenario [4]. Glucose-stimulated insulin secretion from pancreatic beta-cells is controlled by ATP-regulated potassium (KATP) channels, comprised of Kir6.2 and SUR1 subunits [6]. In vitro studies suggest that fluoroquinolones block this channel, resulting in membrane depolarization. This leads to an influx of calcium through voltage-gated calcium channels and a subsequent increase in insulin release, glucose, and hypotonicity-induced cell swelling stimulates an insulin release from pancreatic β-cells but the mechanisms are poorly understood. Recently, Piezo1 was identified as a mechanically-activated nonselective Ca2+ permeable cationic channel in a range of mammalian cells. As cell swelling-induced insulin release could occur through the stimulation of Ca2+ permeable stretch-activated channels, Deivasikamani et al showed that Piezo1 agonist channel induces insulin release from β-cell lines and mouse pancreatic islets could be suggesting a role for Piezo1 in cell swelling-induced insulin release. Hence Piezo1 agonists have the potential to be used as enhancers of insulin release [7]. These similar molecular mechanisms occur when sulfonylureas attach to the sulfonylurea receptor 1 subunit on the KATP channels. This results in the same downstream signaling, resulting in the exocytosis of insulin secretory granules via calcium signaling [8]. In conclusion, fluoroquinolones affect the function of the mitochondria in pancreatic beta cells which may diminish the insulinotropic effect of KATP channel closure and contribute to the hypoglycemic episodes [9].
CONCLUSIONS
This study suggests that levofloxacin can cause hypoglycemia even in nondiabetic and previously healthy patients. The seriousness of hypoglycemia and the few reported deaths also underscore the importance of early detection and appropriate management of patients with hypoglycemia. Taking into consideration the risk of hypoglycemia and other previously identified neuropsychiatric adverse effects [10], clinicians should avoid prescribing fluoroquinolones to patients who have other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections because, for these patients, the risks outweigh the benefits [11].
Consent
The examination of the patient was conducted according to the Declaration of Helsinki principles.
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
REFERENCES
1. Kumar D, Mittal A, Prakash A. Recurrent episodes of hypoglycemia induced by moxifloxacin. Indian J Pharmacol. 2013;45:301-2.
2. Berhe A, Russom M, Bahran F, Hagos G. Ciprofloxacin and risk of hypoglycemia in non-diabetic patients. J Med Case Reports. 2019;13:142.
3. Sullivan M.G, MDedege News. Fluoroquinolones can cause fatal hypoglycemia, FDA warns. FDA/CDC. 2018.
4. Watson M, Ward C, Prabhakar A, Fiza B, Moll V. Successful use of octreotide therapy for refractory levofloxacin-induced hypoglycemia:A case report and literature review. Case Rep Crit Care. 2019;2019:3560608.
5. Morales J, Schneider D. Hypoglycemia. Am J Med. 2014;127:S17-S24.
6. Kharade SV, Sanchez-Andres JV, Fulton MG, Shelton EL, Blobaum AL, Engers DW, et al. Structure-Activity relationships, pharmacokinetics, and pharmacodynamics of the Kir6.2/SUR1-Specific channel opener VU0071063. J Pharmacol Exp Ther. 2019;370:350-9.
7. Deivasikamani V, Dhayalan S, Abudushalamu Y, Mughal R, Visnagri A, Cuthbertson K, et al. Piezo1 channel activation mimics high glucose as a stimulator of insulin release. Sci Rep. 2019;9:16876.
8. Sola D, Rossi L, Schianca GP, Maffioli P, Bigliocca M, Mella R, et al. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11:840-8.
9. Ghaly H, Jörns A, Rustenbeck I. Effect of fluoroquinolones on mitochondrial function in pancreatic beta cells. Eur J Pharm Sci. 2014;52:206-14.
10. Sellick J, Mergenhagen K, Morris L, Feuz L, Horey A, Risbood V, et al. Fuoroquinolone-related neuropsychiatric events in hospitalized veterans. Psychosomatic. 2018;59:259-66.
11. U.S Food and Drug Administration(FDA). FDA reinforces safety information about serious low blood sugar levels and mental health side effects with fouroquinolone antibiotics;requires label changes[safety announcement]. silver spring:FDA;2018.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000_0002_3723_2521 http://orcid.org/0000_0002_3723_2521 http://orcid.org/0000-0002-5145-792X http://orcid.org/0000-0002-5145-792X |



Comments are closed.