Investigating the role of Interleukin-33 and Soluble ST2 in pediatric atopic dermatitis
Ilona Paulauskaite 1, Audrone Eidukaite1,2, Odilija Rudzevičiene2, Rasa Orentaite2
1, Audrone Eidukaite1,2, Odilija Rudzevičiene2, Rasa Orentaite2
1Immunology Department, Centre for Innovative Medicine, Santariški? str. 5, LT-08406, Vilnius, Lithuania. 2Children‘s Hospital, Affiliate of Vilnius University Hospital Santaros Klinikos, Santariški? str. 7, LT-08406, Vilnius, Lithuania
Corresponding author: Dr. Ilona Paulauskaite, E-mail: ilona.paula@mail.com
Submission: 14.10.2019; Acceptance: 15.12.2019
DOI: 10.7241/ourd.20202.2
Cite this article: Paulauskaite I, Eidukaite A, Rudzevičiene O, Orentaite R. Investigating the role of Interleukin-33 and Soluble ST2 in pediatric atopic dermatitis. Our Dermatol Online. 2020;11(2):120-125.
Citation tools:
Copyright information
© Our Dermatology Online 2020. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Background: Atopic dermatitis (AD) is a chronic, inflammatory skin disease, common in children. Pathologic cutaneous inflammation is driven by activated T-helper cells. Studies demonstrate that childhood AD is associated with Th2 immune activation. IL-33 is an intracellular cytokine, abundantly expressed in tissues, which are exposed to the environment. Cellular damage, due to scratching, encounter to infectious pathogens or exposure to allergens, trigger the release of IL-33. Extracellularly IL-33 acts as an activator for Th2 lymphocytes. Soluble ST2 (sST2) is a decoy receptor for IL-33. Combined to sST2, IL-33 loses its biological functions, which results in the alleviation of Th2 immune response. With this study we wanted to investigate the role of IL-33 and sST2 in pediatric AD.
Material and Methods: Blood and stool samples from children with AD and healthy controls were tested for IL-33 and sST2 concentrations.
Results: Children with AD presented significantly higher blood IL-33 concentrations, compared to healthy controls: 18,21 pg/ml vs 0, p<0,05. Stool IL-33 levels demonstrated no significant difference between the two groups: 12,43 pg/ml vs 45,94 pg/ml, p>0,05. Blood and stool sST2 concentrations showed no significant difference: 67,58 pg/ml vs 74,96 pg/ml, p>0,05; 0 vs 0, p>0,05, respectively.
Conclusion: IL-33 is associated with pediatric AD. Blood, but not stool IL-33 testing can be used as a biomarker. sST2 showed no difference in AD.
Key words: Atopic Dermatitis; Interleukin-33; Soluble ST2 Receptor
INTRODUCTION
Atopic dermatitis (AD) is a complex inflammatory cutaneous disorder characterized by immune-mediated inflammation and epidermal barrier dysfunction [1]. AD has a complex etiology, it develops in early childhood and has age dependent distribution [2]. AD affects approximately 20% of children and up to 3% of adults, data shows that it’s prevalence is still increasing [3]. Most of the AD end up in remission, small number of childhood cases, especially severe ones persist into adulthood [4]. Studies show, that AD predispose to a higher risk of atopic and other than atopy comorbidities [5,6].
AD is heterogeneous in its pathophysiological pathways. Studies demonstrate that different T-helper subsets and cytokines drive pathological cutaneous inflammation [7]. Children with AD have a dominant Th2 activation and expansion [8]. Determining the correct cellular subset could have a potential in not only providing a better understanding in AD pathophysiology, but also be a potential diagnostic and prognostic tool [9].
Interleukin-33 (IL-33) is a member of interleukin-1 family cytokines, it is found in the nuclei of various tissue and immune cells in human body [10]. IL-33 is highly expressed in barrier tissues, which are exposed to the environment (e.g., skin, gut, lungs), those tissues are considered to be the major sources of IL-33 in human body [11]. IL-33 extracellularly can be detected following cellular damage or stress due to infection, allergen exposure [12]. IL-33 expression can be induced by inflammation, environmental triggers [13]. IL-33 activates Th2 lymphocytes, promotes IL-4, IL-5, IL-13 cytokine secretion, acts as a chemoattractant for Th2 lymphocytes [14,15]. Soluble serum stimulation 2 receptor (sST2) is an alternative splice variant of the gene, encoding ST2. Combined to sST2 IL-33 loses its biological functions, which results in the alleviation of Th2 immune response [16].
Data, concerning pediatric AD and prospective biomarkers is lacking. With this study we wanted to evaluate the role of IL-33/sST2 axis in pediatric AD.
MATERIALS AND METHODS
Study Population and Ethical Considerations
A total of 68 children were invited to take part in the study. Study population comprised of 51 participants with clinically proved atopic dermatitis, hospitalized in Children’s Pulmonology and Allergology Department and 17 healthy control subjects, who had no history of atopic diseases, nor any current inflammatory diseases.
Sample Collection and Laboratory Analysis
Venous blood and stool samples were obtained from test and control subjects. All the samples were collected as part of the routine clinical practice. Automated blood test, allergen specific IgE tests were performed at once. Complete blood count was obtained from venous blood using automated hematology analyzer (Sysmex XT 4000i, Roche, Germany). Allergen specific IgE tests were performed using Phadia Immunocap 100 analyzer (Phadia, Uppsala, Sweden). The rest of the samples were stored at -80°C for further IL-33 and sST2 testing.
Measurement of Serum and Fecal IL-33, sST2
Frozen stool and serum samples were completely defrosted prior testing. Suspensions were prepared from stool samples: 0,1 g stool was suspended in 1 ml phosphate saline buffer (PBS, pH=7,2). Suspensions were thoroughly vortexed, left to sit at room temperature for 15 min, then once again vortexed and centrifuged (10000 x g, 20 min). Supernatants were used for the test procedure. Supernatants and serum samples were tested for IL-33 using Human IL-33 ELISA kit (Elabscience, China), sST2 – Human IL-1 R4 (IL1RL1) ELISA kit (Thermo Fisher Scientific, USA). Assay procedures were performed according to manufacturer’s recommendations.
Data Management and Statistical Analysis
MS Office Excel, MedCalc software were used for data management and statistical analysis. Nonparametric data were expressed with median and range. Mann-Whitney U test was used for compared two groups of variables. Categorical data were expressed with a number and percentage, difference was determined using Chi-Square test. Difference between the groups was considered significant when p<0,05.
Ethics Statement
Participants parents or legal guardians provided their agreement in participating in the study by signing a written informed consent form. Ethics approval for the research study was obtained (No. 158200-16-834-352).
RESULTS
Baseline Characteristics
Test and control subjects were a match according to age and gender (Table 1). Total leukocyte count, absolute neutrophil and eosinophil counts in venous blood were measured. Test subjects were tested for food specific IgE. 43% (n=22) had non detectable food specific IgE, 57% (n=29) were sensitized to food (food specific IgE ≥ 0,35 kUA/l). Sensitization was mostly detected to cow‘s milk and hen‘s egg. Detailed information is provided in Table 1.
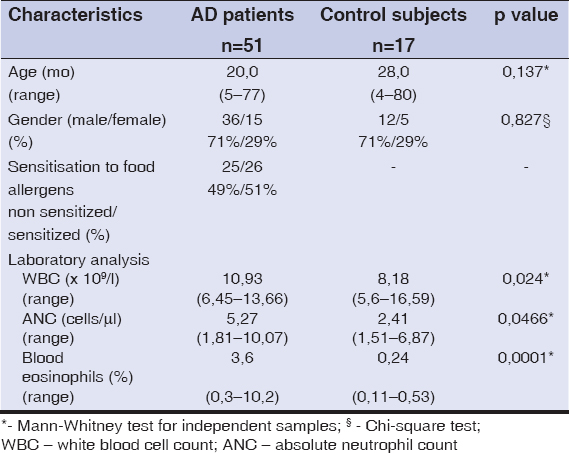 |
Table 1: Patient characteristics |
IL-33 and sST2 Concentrations
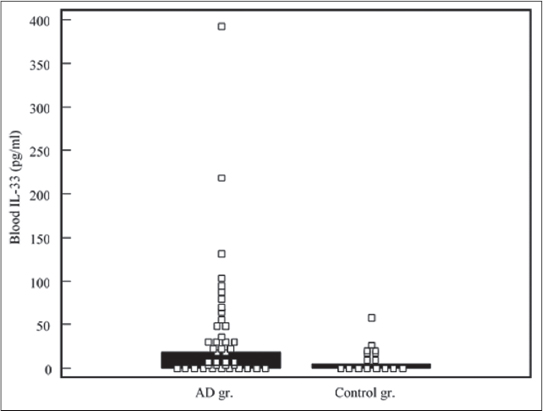
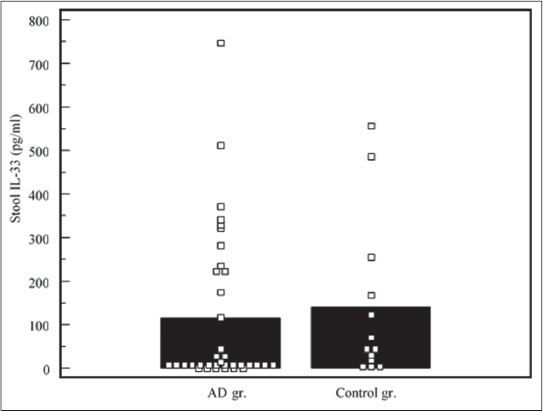
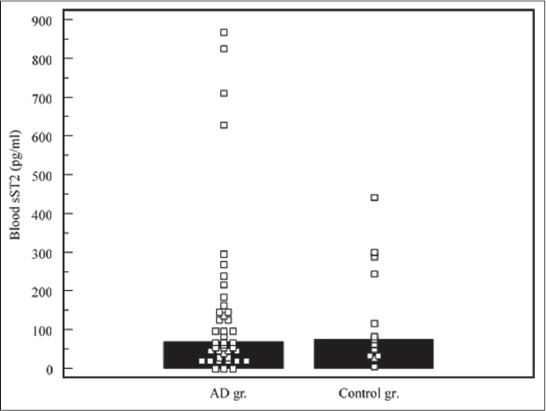
Children with AD had higher blood IL-33 levels (median: 18,21 pg/ml, range: 0 – 392,89 pg/ml), compared to controls (median: 0 pg/ml, range: 0 – 58,31 pg/ml), the difference was significant, p<0,05 (Fig. 1). Stool IL-33 concentrations in test subjects (median: 12,43 pg/ml, range: 0 – 745,56) was lower, compared to controls (median: 45,94 pg/ml, range: 3,43 – 556,77 pg/ml), the difference was not significant, p>0,05 (Fig. 2). sST2 levels demonstrated similar distribution. Median of blood sST2 in test subjects was 67,58 pg/ml (range: 0 – 867,70 pg/ml), controls – 74,96 pg/ml (range: 3,77 – 441,82 pg/ml), the difference was not significant, p>0,05 (Fig. 3). Stool sST2 concentration both in test and control groups was 0, test group ranges: 0 – 117,78 pg/ml, control group: 0 – 210 pg/ml, there was no difference between the groups, p>0,05 (Table 2, Fig. 4).
 |
Table 2: Blood and stool IL-33 and sST2 concentrations |
 |
Figure 4: Stool sST2 concentrations Comparison of stool sST2 concentrations in atopic dermatitis (AD) and control groups. Medians are marked with black bars. |
DISCUSSION
AD is a common, chronic, relapsing, inflammatory skin disease primary affecting young children [17]. Experimental models with transgenic mice, grown under pathogen-free conditions, demonstrate that IL-33 over expression in the skin resulted in spontaneous itchy dermatitis [18]. Human studies also demonstrate, that IL-33 expression is significantly increased in the lesional skin [19]. IL-33 signaling is crucial in the development of experimental models of AD [20]. Mechanical damage, due to scratching, infection, exposure to allergens, triggers the release of IL-33 [21]. C. Galand et al. in his experimental murine model demonstrates that serum IL-33 levels were significantly increased after a tape stripping experiment [22]. In our study children with AD had significantly higher serum IL-33 concentration compared to controls (18,52 pg/ml vs 0 pg/ml, p<0,05). R. Tanagawa-Mineoka et al. reports, that IL-33 was significantly higher in patients with AD compared to patients with chronic idiopathic urticaria, psoriasis and healthy controls [23]. AD is a multifactorial disease, its main pathogenetic factor is immune mediated inflammation. IL-33 is associated with Th2 immune activation. According to T. Czarnowicki et al. children with AD had a markedly expanded Th2 type lymphocyte population in their blood [8]. Other studies also present data that AD is associated with an expansion and activation of Th2 lymphocytes in peripheral blood [24]. U. Nyagaard et al. demonstrates, that serum levels of IL-33 and sST2 were elevated in adults and children with AD compared to healthy controls [25]. The researchers also found that serum IL-33 levels were much higher in children compared to adults with AD. On the contrary, adults with AD presented with significantly higher sST2 values compared to children with AD. This could mean that there might be an age dependent distribution of IL-33 and sST2. Although available data are missing, this should be taken in consideration comparing studies with children and adults.
IL-33 biological functions manifest through its receptor – ST2. It exists in two main isoforms: a membrane bound (ST2L) and soluble (sST2) [26]. Small intestine, heart, kidney, lung tissues display the highest expression and are considered to be the main sources of sST2 in human body [27]. Immune cells normally do not secrete sST2, but under certain conditions they can also be a significant source of sST2 [28]. We did not detect any differences in blood sST2 concentrations compared AD patients to controls: 67,57 pg/ml vs 78,58 pg/ml, p>0,05. Relationship between IL-33 and sST2 in is still under investigation. Studies demonstrate that both IL-33 and sST2 were elevated in asthmatic children [29]. There are not many data, concerning AD. U. According to Nygaard et al. increased IL-33, but not sST2 levels, were associated with AD. sST2 did not correlate with disease activity [25]. According to literature, inflammatory conditions activate sST2 synthesis and secretion in tissue cells. D. Diaz-Jimenez et al. states, that increased sST2 concentration reflected inflammatory activity in patients with ulcerative colitis [30]. It is not clear, whether sST2 provides negative regulation for the exacerbation of IL-33 biological functions in the pathogenesis of AD and what could influence those processes. P. E. Pfeffer et al. investigated the effect of vitamin D on IL-33/sST2 axis in experimental cell model. According to the researchers, vitamin D selectively upregulated sST2 expression and impeded IL-33 biological functions [31]. Although, vitamin D affects multiple systems, evidence suggests that it has a beneficial effect on AD course [32].
Recently, there has been a growing interest in gut microbiome and its possible immunomodulatory effect on systemic disorders [33]. Evidence supporting the gut-skin axis is inconclusive, but there are studies demonstrating that alterations in microbiome might be a triggering signal for the dysregulation of the immune response [34]. As well as epithelial cells in the skin, gastrointestinal tract enterocytes can also be a significant source of IL-33 [35]. Exposure of the intestinal epithelial cells to food allergens increases IL-33 expression [36]. Hoewer, there are still questions remaining about its active secretion. Half of our test subjects were sensitized to food. Stool testing for IL-33 and sST2 could reflect the immune reactions ongoing in the gut mucosa. It has a potential as a noninvasive method. However, we found no significant difference in stool IL-33 concentrations compared patients with AD and healthy controls: 14,11 pg/ml vs 57,83 pg/ml, p>0,05. Stool sST2 levels also demonstrated no difference between the groups. Literature provides no data concerning stool testing for IL-33 and sST2. According to J. Penders et al. study, intestinal microbiota plays an important role in the development of AD in early childhood [37]. An experimental murine model demonstrates that germ-free mice fail to develop oral tolerance, presence of gut microbiota have a protective role against AD formation [38,39]. According to our study, stool testing did not reveal any activation of IL-33/sST2 axis in AD patients, further testing is needed.
CONCLUSION
In conclusion: the increase in IL-33 concentration is associated with pediatric AD. Blood, but not stool IL-33 testing can be used as a biomarker. sST2 showed no difference in pediatric AD, further investigations are needed.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent
Informed consent was obtained from all patients for being included in the study.
Copyright by Ilona Paulauskait?, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
REFERENCES
1. Guttman-Yassky E, Waldman A, Ahluwalia J, Ong PY, Eichenfield LF. Atopic dermatitis:pathogenesis. Semin Cutan Med Surg. 2017;36:100-3.
2. Lyons JJ, Milner JD, Stone KD. Atopic dermatitis in children:clinical features, pathophysiology and treatment. Immunol Allergy Clin North Am. 2015;35:161-83.
3. Flohr C, Mann J. New insights into epidemiology of childhood atopic dermatitis. Allergy. 2014;69:3-16.
4. Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD):A systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681-7.
5. Binyamin ST, Algamal F, Yamani AN, Labani MS, Alaqbi FH, Baeshen AA, et al. Prevalence and determinants of eczema among females aged 21 to 32 years in Jeddah city – Saudi Arabia. Our Dermatol Online. 2017;8:22-6.
6. Lenga Loumingou IA, Loumingou R. Meaning of microalbuminuria during the atopic dermatitis of the child. Our Dermatol Online. 2019;10:251-4.
7. Wang A, Landen NX. New insights into T cells and their signature cytokines in atopic dermatitis. IUBMB Life. 2015;67:601-10.
8. Czarnowicki T, Esaki H, Gonzalez J, Malajian D, Shemer A, Noda S, et al. Early pediatric atopic dermatitis shows only a CLA+Th2/Th1 imbalance, while adults acquire CLA+Th22/Tc22 subsets. J Allergy Clin Immunol. 2015;136:941-51.
9. Ungar B, Garcet S, Gonzalez J, Dhingra N, Correa da Rosa J, Shemer A, et al. An integrated model of atopic dermatitis biomarkers highlights the systemic nature of the disease. J Invest Dermatol. 2017;137:603-13.
10. Carrasco TG, Morales RA, Pérez F, Terraza C, Yáñez L, Campos-Mora M, et al. Alarmin’immunologists:IL-33 as a putative target for modulating T cell-dependent responses. Front Immunol. 2015;6:1-8.
11. Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676-89.
12. Cayrol C, Girard JP. IL-33:An alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31-7.
13. Gautier V, Cayrol C, Farache D, Roga S, Monsarrat B, Gonzalez De Peredo A, et al. Extracellular IL-33 cytokine, but not endogenous nuclear IL-33, regulates protein expression in endothelial cells. Sci Rep. 2016;6:1-12.
14. Murakami-Satsutani N, Ito T, Nakanishi T, Inagaki N, Tanaka A, Vien PT, et al. IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol Int. 2014;63:443-55.
15. Komai-Koma M, Xu D, Li Y, McKenzie A, McInnes IB, Liew FY, et al. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37:2779-86.
16. Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol. 2017;8:1-17.
17. Thomsen SF. Atopic dermatitis:natural history, diagnosis, and treatment. ISRN Allergy. 2014;2014:1-7.
18. Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutami H, Yoshimoto T, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U.S.A. 2013;110:13921-6.
19. Abdel Hay RM, Ibrahim NH, Metwally D, Rashed LA. The role of interleukin-1ßand interleukin-33 in atopic dermatitis. Our Dermatol Online. 2013;4:11-4.
20. Li C, Maillet I, Mackowiak C, Viala C, Padova F, Li M, et al. Experimental atopic dermatitis depends on IL-33R signaling via MyD88 in dendritic cells. Cell Death Dis. 2017;8:1-11.
21. Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimäki S, et al. IL-33 and ST2 in atopic dermatitis:Expression profiles and modulation by triggering factors. J Investig Dermatol. 2012;132:1392-400.
22. Galand C, Leyva-Castillo JM, Yoon J, Han A, Lee MS, McKenzie ANJ, et al. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol. 2016;138:1356-66.
23. Tamagawa-Mineoka R, Okuzawa Y, Masuda K, Katoh N. Increased serum levels of interleukin 33 in patients with atopic dermatitis. J Am Acad Dermatol. 2014;70:882-8.
24. Czarnowicki T, Gonzalez J, Shemer A, Malajian D, Xu H, Zhenh X, et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol. 2015;136:104-15.
25. Nygaard U, Hvid M, Johansen C, Buchner M, Fölster-Holst M, Deleuran M, et al. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol. 2016;30:1930-8.
26. Zhao Q, Chen G. Role of IL-33 and its receptor in T cell-mediated autoimmune diseases. Biomed Res Int. 2014;2014:1-10.
27. Mildner M, Storka A, Lichtenauer M, Mlitz V, Ghannadan M, Hoetzenecker K, et al. Primary sources and immunological prerequisites for sST2 secretion in humans. Cardiovasc Res. 2010;87:769-77.
28. Zhang J, Ramada AM, Griesenauer B, Li W, Turner MJ, Liu C, et al. ST2 blockade reduces sST2-producing T cells while maintaining protective mST2-expressing T cells during graft-versus-host disease. Sci Transl Med. 2015;7:1-27.
29. Chu MA, Lee HJ, Lee EJ, Hong SJ, Park HJ, Lee KH, et al. Increased serum soluble ST2 in asthmatic children and recurrent early wheezers. Allergy Asthma Respir Dis. 2013;1:314-20.
30. Díaz-Jiménez D, De la Fuente M, Dubois-Camacho K, Landskron G, Fuentes J, Pérez T, et al. Soluble ST2 is a sensitive clinical marker of ulcerative colitis evolution. BMC Gastroenterol. 2016;16:1-10.
31. Pfeffer PE, Chen YH, Woszczek G, Matthews NC, Chevretton E, Gupta A, et al. Vitamin D enhances production of soluble ST2, inhibiting the action of IL-33. J Allergy Clin Immunol. 2015;135:824-7.
32. Filippo PD, Scaparrotta A, Rapino D, Cingolani A, Attanasi M, Petrosino MI, et al. Vitamin D supplementation modulates the immune system and improves atopic dermatitis in children. J Allergy Clin Immunol. 2015;166:91-6.
33. Lee SY, Lee E, Park YM, Hong SJ. Microbiome in the gut-skin axis in atopic dermatitis. Allergy Asthma Immunol Res. 2018;10:354-62.
34. Vaughn AR, Notay M, Clark AK, Sivamani RK. Skin-gut axis:The relationship between intestinal bacteria and skin health. World J Dermatol. 2017;6:52-8.
35. Pascual-Reguant A, Bayat Sarmad J, Baumann C, Noster R, Cirera-Salinas D, Curato D, et al. TH17 cells express ST2 and are controlled by the alarmin IL-33 in the small intestine. Mucosal Immunol. 2017;10:1431-42.
36. Starkl P, Krishnamurthy D, Szalai K, Felix F, Oberthuer D, Sampson HA, et al. Heating affects structure, enterocyte adsorption and signalling, as well as immunogenicity of the peanut allergen ara h 2. Open Allergy J. 2011;4:24-34.
37. Penders J, Gerhold K, Stobberingh EE, Thijs C, Zimmermann K, Lau S, et al. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol. 2013;132:601-7.
38. Rodriguez B, Prioult G, Bibiloni G, Nicolis I, Mercenier A, Butel MJ, et al. Germ-free status and altered caecal subdominant microbiota are associated with a high susceptibility to cow’s milk allergy in mice. FEMS Microbiol Ecol. 2011;76:133-44.
39. Zachariassen LF, Krych L, Engkilde K, Nielsen DS, Kot W, Hansen CHF, et al. Sensitivity to oxazolone induced dermatitis is transferable with gut microbiota in mice. Sci Rep. 2017;7:1-11.
Notes
Source of Support: Nil.
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|



Comments are closed.