|
Get Citation
|
|
|
Sokolova TV, Adaskevich UP, Malyarchuk AP, Lopatina YV. Scabious erythroderma – a rare clinical variant of scabies. Our Dermatol Online. 2018;9(4):355-362. |
|
|
Download citation file:
|
Scabious erythroderma – a rare clinical variant of scabies
Tatyana V. Sokolova1, Uladzimir P. Adaskevich2, Alexander P. Malyarchuk1, Yulia V. Lopatina3
1Department of Skin and Venerial Diseases and Cosmetology, Medical Institute of Post-Graduate Education, Federal State Educational Institution of Higher Professional Education “Moscow State University of Food Production”, Moscow, Russian Federation, 2Department of Dermatovenereology, Vitebsk State Medical University, Vitebsk, Belarus, 3Department of Entomology, Biological Faculty, M.V. Lomonosov Moscow State University, Moscow, Russian Federation
Corresponding author: Prof. Uladzimir P. Adaskevich, E-mail: vitebsk.derma@mail.ru
Submission: 16.01.2018; Acceptance: 10.05.2018
DOI: 10.7241/ourd.20184.1
ABSTRACT
Background: Erythroderma (exfoliative dermatitis) is an emergency condition in dermatology in which not less than 90% of skin surface is affected. The presenting features are erythema, skin scaling and itching, fever and lymphadenopathy. The most common cause of erythroderma is a preexisting dermatosis (psoriasis, atopic dermatitis, eczema, seborheic dermatitis, lichen rubra pilaris, lichen planus, pemphigus foliaceous), drug reactions, lymphoma, leukemia and visceral neoplasias. Erythroderma is a diagnostically relevant presenting feature of Norwegian scabies.
The aim of investigation: Is to describe clinical peculiarities of scabious erythroderma as a special rare form of scabies, to assess the number of scabies mites on the patient and in his/her environment and to work out the criteria of differential diagnosis with Norwegian scabies.
Material and methods:: We examined 5 patients with scabies and erythroderma as the main presenting feature. All patients were women aged from 42 to 89 years. The disease duration was from 8 months to 1 year. The causes of erythroderma were variable. Clinical and paraclinical methods of investigation alongside with dermatoscopy and microscopy were used.
Results: This is the description of a rare clinical form of scabies, scabious erythroderma. It is based on the analysis of the 5 cases of scabies, whose main clinical manifestation is diffuse erythroderma. The diagnostic criterias of scabious erythroderma and differential diagnosis of Norwegian scabies are given. The invasive potential of this form of the disease on the patient and beyond is evaluated for the first time.
Key words: Scabious erythroderma; Norwegian scabies; Dermatoscopy; The differential diagnosis
INTRODUCTION
Erythroderma is an inflammatory skin condition characterized by erythema and exfoliative dermatitis involving 90% and more of the entire skin surface. The initial lesions which are important keys for understanding the disease evolution are often occult [1]. The most common causes of erythroderma can include pre-existing dermatoses (psoriasis, atopic dermatitis, eczema, seborrhoeic dermatitis, lichen ruber pilaris, lichen ruber planus, pemphigus foliaceus, bullous pemphigoid), drug-induced eruption, lymphoma and leukemia, visceral neoplasias and other conditions [1–4].
Erythroderma is also a diagnostically relevant clinical manifestation of Norwegian scabies [1,5,6]. The latter was first described by Danielson and Boeck in Norway in 1848. Crusted scabies is another term used to name this condition. This name reflects the main clinical symptom of the disease – massive crusts which are formed in various areas of the skin surface. In addition to crusts and erythroderma, Norwegian scabiesis characterized by multiple burrow tracks, polymorphous eruption (papules, vesicles, pustules) and scales.
The etiology and peculiarities of the disease evolution have been quite competently systemized [5,7]. For the last two decades the cases of Norwegian scabies have been described in HIV-infected patients [8–11], in elderly and disabled people [12,13] and rarely observed in cases of brain astrozytoma [14], drug addiction [15], Down syndrome, diffuse fatty liver disease, anemia, parenchymatous dystrophy of visceral organs, cachexia [16], bullous pemphigoid treated with systemic c orticosteroids [17], congenital erythroderma [18], in patients taking novel immunosuppressive agents tozilisumab [19] and cyclosporine [20], in case of skin exposure to pesticides [21]. Rare cases of Norwegian scabies are also described without associated pathology: in a 24-year-old man [22], in a pregnant woman [23], in children [24,25].
Massive crusts are the main symptom of Norwegian scabies. Their thickness varies from several millimeters to 2-3 cm. In some cases crust layers may cover considerable areas of the skin surface forming a solid horny shield which limits body movements and makes thempainful. The crust colour varies greatly from dirty gray with a mixture of blood to yellowish-green, grayish-brown or alabaster-white. The crust surface is rough, fissured and covered with verrucous rupia-like proliferations. Crusts usually appear at the preferable sites of burrows (hands, feet, elbows, buttocks and other localizations). The upper crust layer is firm, the lower one is friable. Between these two layers a great amount of adult and immature mites can be found. On the inner crust surface one can see tortuous depressions which correspond to scabies mite burrows. The crusts firmly adhere to the skin surface and, if forcibly removed, leave large weeping erosions. The burrows with in the crusts are “many-storied”. In the lower crustose layers, male and female mites, nymphs, larvae and eggs can be detected, and in the deep inner layers, dead mites and eggs, as well as empty egg shells are found. The number of mites on a sick patient is immense, so the Norwegian scabies is highly contagious with local epidemics breaking out around the patient.
Erythroderma is the second diagnostically relevant symptom of Norwegian scabies [6,13,16,26–31]. The cause of erythroderma in this case is considered to be Staphylococcus aureus colonizing mite burrows [32,33]. Staphylococcus aureus was found in mite burrows of an elderly patient with Norwegien scabies by scanning electron microscopy, bacterial analysis of burrow contents revealed Staphylococcus aureus and Staphylococcus haemolyticus. [34]. It is important to note the observation suggesting that erythroderma in Norwegian scabies arising on the background of both systemic and topical corticosteroid therapies appears earlier than in case when corticosteroid therapy is not administered [35,36].
Other diagnostically relevant criteria of Norwegian scabies are affected nails (nailplates easily crumble, they are grey with abumpy surface and not chededges, sometimes nail plates are completely lost and replaced by massive epidermal crustlike layers); enlargement of multiple lymph nodes (polyadenopathy); fever during the entire course of the disease; palmar-plantar hyperkeratosis; hair changes (dry, dull, ash-gray) up to alopecia; body malodour (reminiscent of sour dough) [6,26–31,37].
There are some case reports in medical literature describing highly contagious scabies with extensive erythroderma as the main clinical symptom [38,39]. This rare erythrodermic form of scabies is still insufficiently described in medical literature. That is why some authors, having found areas of hyperkeratosis (which are no crusts actually), diagnose such cases as Norwegian scabies [38–42]. In fact, the given form of the disease should be designated as scabious erythroderma. One can assume that there must be far more similar cases. Besides, it is recognized that Norwegian scabies may have a localized form with crusts developing only in certain areas of the skin surface [7,41,42].
The aim of our study was to describe peculiarities of the clinical course of scabious erythroderma as an independent rare variant of scabies, to estimate the number of mites on patients and in their surroundings and to work out criteria of differential diagnostics with Norwegian scabies.
MATERIALS AND METHODS
We observed 5 patients with scabies in whom erythroderma was the main clinical manifestation of the disease. All patients were women aged 42, 72, 76, 84 and 89. The duration of the disease was from 8 months to 1 year. The causes of the disorder were different in all the patients. The condition in the first patient (aged 42) developed on the background of systemic lupus erythematous. The complex therapy of this disease included prednisolone 60 mg/day during 3 months. In two patients (aged 72 and 76) allergic contact dermatitis and then drug-induced reaction were erroneously diagnosed. During 8-9 months the patients received systemic antihistamine, desensitizing drugs and topical glucocorticosteroids. Erythroderma appeared two months after topical application of corticosteroids. The forth case was a 84-year-old patient of psychoneurologic department. Many years the patient took systemic psychotropic drugs for schizophrenia, fluocinolon acetonide was applied topically. The fifth patient (aged 89), in whom allergic dermatitis and then drug-induced reaction were diagnosed, received systemic antihistamine drugs, topical corticosteroids during one year and then three-month course of betamethasone. Erythroderma appeared after 2 months of betamethasone injections.
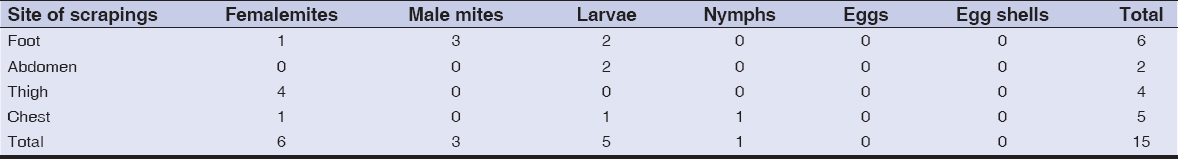
In all cases the diagnosis of scabies was confirmed by laboratory investigations. The laboratory methods included mite removal with the help of a needle, burrow and lesional skin scrapings with lactic acid application, dermatoscopy performed with the help of the dermatoscope DELTA 20 and microscopy with USB-microscopes of various modifications. The number of burrows was counted visually and by means of dermatoscopy and then the parasitary index was determined. In the fifth patient the number of mites on the apparently normal skin and in erythrodermic lesions was counted in the field of a standard dermatoscope with the area of 1 cm2. The efficacy of scabies diagnostics by means of dermatoscopy and tape-test methods [43,44] was compared. In case of a tape test, a piece of transparent adhesive Scotch tape (2×5 cm) was applied on an affected site of the skin for several seconds and then quickly removed. The removed piece of tape was paced on the slide and viewed with the microscope. The quantities of mites in different stages of development were compared in two epidermal scrapings (from the abdomen and thigh) and in 4 Scotch-tests (from the foot, chest, back, thigh). The number of mites around the patient was determined on the sheet where the patient was lying. For this purpose the adhesive tape (2×5 cm) was applied to ten different sites on the sheet.
As an example we describe a case of a patient with scabious erythroderma diagnosed in June 2013 (Figs. 1–6). A 89-year-old patient admitted to hospital complained of the affection of the whole skin, moderate itch increasing in the evening and chills (in spite of high environmental temperature). The disease had lasted for one year. The patient did not connect any events with the onset of the disease and considered the skin changes to be a result of “allergy” (she had previously worked as a nurse). The first symptom of the disease was itch in the interscapular region. The itching sensation then gradually spread to other skin regions. The patient’s daughter who cared for her mother also complained of slight itch. Both women took antihistamine drugs and applied topical medicines against pruritus with no effects after this self-treatment. On admission to hospital the condition of the old patient was diagnosed as wide-spread allergic dermatitis. The patient was treated with antihistamine and desensitizing drugs and topical corticosteroid creams. Short-termin significant improvement was observed. The patient applied to 4 different doctors but the diagnosis remained the same and the treatment did not significantly differ from the previous one. The therapeutic measures brought no effect. Three months before admission to hospital the patient was administered 2 injections of betamethasone per month and topical corticosteroid creams (clobetasol, fluticasone). While the subjective perception of itch reduced, there appeared lesions of erythema which quickly spread and covered the whole skin surface creating the clinical picture of erythroderma. With the diagnosis “drug-induced eruption” the patient was admitted to hospital. The patient’s condition on admission was satisfactory, the body temperature was normal. The state of the inner organs and the revealed pathology in general corresponded to the advanced age of the patient. The regional lymphnodes were painless and not enlarged.
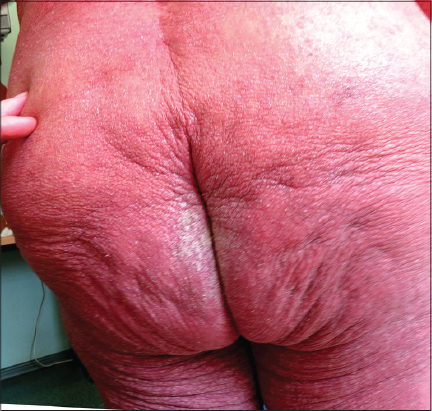
Local status. The process was of a universal character with erythroderma covering the whole skin surface of the body. The skin was dusky red, dry and in some areas scaling with signs of infiltration, pigmentation and lichenification. The skin felt warm, firm and rough. White dermographism was observed, but crusts were absent. In the areas of the intergluteal cleft (Fig. 1) and elbows there were foci of grey hyperkeratosis with firmly adherent scales. Scratch marks were hardly present. On the background of moderate facial hyperemia there were red infiltrated lesions on the forehead, chin, eyelids, ears, cheeks, and the vermillion border of the lips. The skin of the scalp was pale without any signs of inflammation. On the skin of the shoulders and lower legs there were small isles of normal skin. The inflammatory changes of the palmar and plantar surfaces were insignificant. Multiple fresh and destroyed burrows of various lengths were observed predominantly in the skin folds. The number of burrows detected without using a dermatoscope made up 186 on the palms, 81 on the soles and 34 on the areolas. Burrows in other skin regions were poorly visualized without a dermatoscope.
Laboratory data. Moderately elevated WBC count (13,6×109/L), ESR 3 mm/h, hypoproteinemia 52 g/L. Other blood biochemistry values and urine analysis were within the normal range.
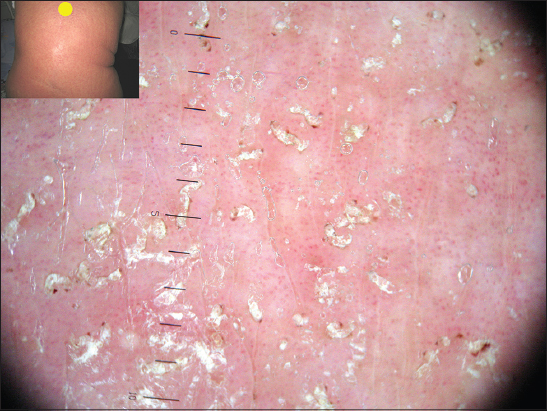
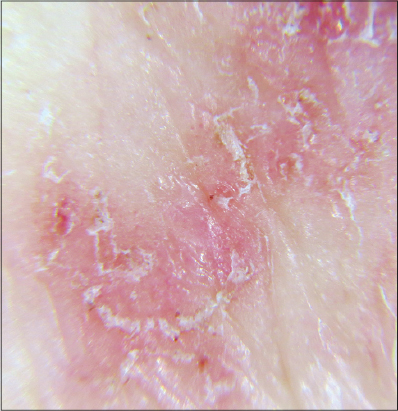
Dermatoscopy. In all areas of the skin surface multiple mite burrows were found, including the face, frontal hairline on the head (Fig. 2), interscapular (Fig. 3) and pubic (Fig. 4) regions. Mites (from 5 to 30 on 1 cm2) were detected beyond the burrows (Fig. 4) even in only slightly changed areas (Fig. 5). The number of mites was the biggest in those areas of the skin surface where the inflammatory changes were the most dramatic ones. In order to compare the effectiveness of visual and dermatoscopic methods for detecting mites, the parasites were counted on the palm skin surface with the area of 4 cm2. Only female mites located in the burrows were visually detected (total 19). Twice as many mites (total 41) were found by means of dermatoscopy, including parasites beyond the burrows. Microscopy of skin scrapings yielded the following results (Table 1).
The number of parasitic elements clearly depended on the size of the skin area to be scraped. In scrapings from the thigh (6×8 cm) 17 parasitic elements were revealed and in scrapings from the abdomen the number of such elements was 25. Adult mites (male and female) prevailed (40,5%), eggs made up 33,3% of all parasitic elements, empty egg shells and larvae accounted for 19,1% and 7,1% of elements, respectively. The obtained data show a high level of mite colonization in those areas of the skin surface which are only insignificantly affected in case of common scabies. The prevalence of female mites and empty egg shells in skin scrapings speaks for the presence of such burrows which, in case of common scabies, are usually found on the hands, wrists and feet.
For the diagnosis of scabies tape-tests were used. Their results are given in Table 2.
By means of tape-tests taken from 4 sites of the skin surface 15 mites in various stages of development were found. The adult mites (imago) dominated including 6 female and 3 male mites. Larvae (6) and nymphs (1) were also detected, but there were no eggs or egg shells. Mites were found not only in sites of typical burrow localization (feet) but also in those areas of the skin surface where, in case of common scabies, elements of metamorphic stage of the life cycle are localized (abdomen, thigh, chest).
While comparing the effectiveness of dermatoscopy and tape-test methods a considerable advantage of dermatoscopy was evident. The number of mites revealed in 4 tape-tests on the area of 10 cm2 varied from 2 to 6 parasites. Dermatoscopy of the site with the same area revealed 35 mites on the foot, 12 on the abdomen, 22 and 15 on the thigh and chest, respectively.
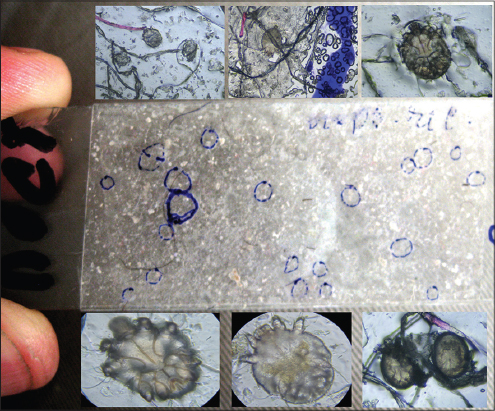
Three tape-tests were made with the sheet on which the patient was lying (Fig. 6 a, b) which revealed 9 female mites, 6 male mites, 11 larvae, 1 nymph and 4 eggs. These results speak for a high invasive potential of this scabies form. All family members who cared for the patient also had scabies.
With regard to clinical, dermatoscopic and microscopic data the diagnosis of scabies in the form of scabious erythroderma was made. The patient was treated with benzyl benzoate ointment 20%. The next day the efficacy of the treatment was assessed in terms of mobility of parasites. Mobility was observed in 67% of mites extracted from the burrows and in 92% of mites removed from the apparently normal skin. In tape-tests taken from the patient’s sheet 23 mites of 27 (85,2%) retained their mobility. Benzyl benzoate ointment was applied on the whole skin surface once a day in the evening for 7 days. Simultaneously, loratadine was administered. On day 8 a significant reduction of infiltration and hyperemia was observed and the number of mites on dermatoscopy decreased to 1-3 per cm2, no mobile mites were found. Tape tests taken from the skin surface and the sheets were negative. The following therapy included desensitizing (sodium thiosulfate) and antihistamine (chlorpheniramine) drugs, as well as topical application of emollients (cold cream).
DISCUSSION
The analysis of the 5 clinical cases allows scabious erythroderma to be singled out as a separate rare form of scabies. The clinical diagnostic criteria of this form are as follows:
- Development of the disease on the background of taking medicines which reduce itch, such as systemic and topical corticosteroids, psychotropic, antihistamine and desensitizing drugs. The suppression of itch reduces scratching thus preserving mites in the skin. So the population of mites is uncontrolledly increasing.
- Considerable duration of the disease (> 8 months) with early erythroderma appearing 2-3 months after administering systemic and topical corticosteroids, often in combination with antihistamine and/or psychotropic drugs.
- Peculiar character of itch: less severe, diffuse, increasing in the evening, without scratch marks. Patients usually do not scratch but rather rub the skin with their hands.
- Generalized erythema with infiltration (erythroderma) and xerosis with minimal scaling.
- Areas of hyperkeratosis on the sites of constant pressure (buttocks, elbows).
- Crusts are absent.
- Presence of only small pustules with slight infiltration at the base (osteofolliculitis).
- A great number of burrows at the sites of preferable localization (hands, wrists, feet): 50-310 in an anatomic region.
- Presence of burrows on the face, neck and in the interscapular region where they are usually absent in case of common scabies.
- Prevalence of the so-called metamorphic burrows (2-3 mm long) which are mostly made by immature parasites (larvae, nymphs) [5].
- Persistent white dermographism.
- Mites are visualized by dermatoscopy not only in burrows but also on erythrodermic as well as on apparently normal skin.
- All persons in contact with the patient are infested.
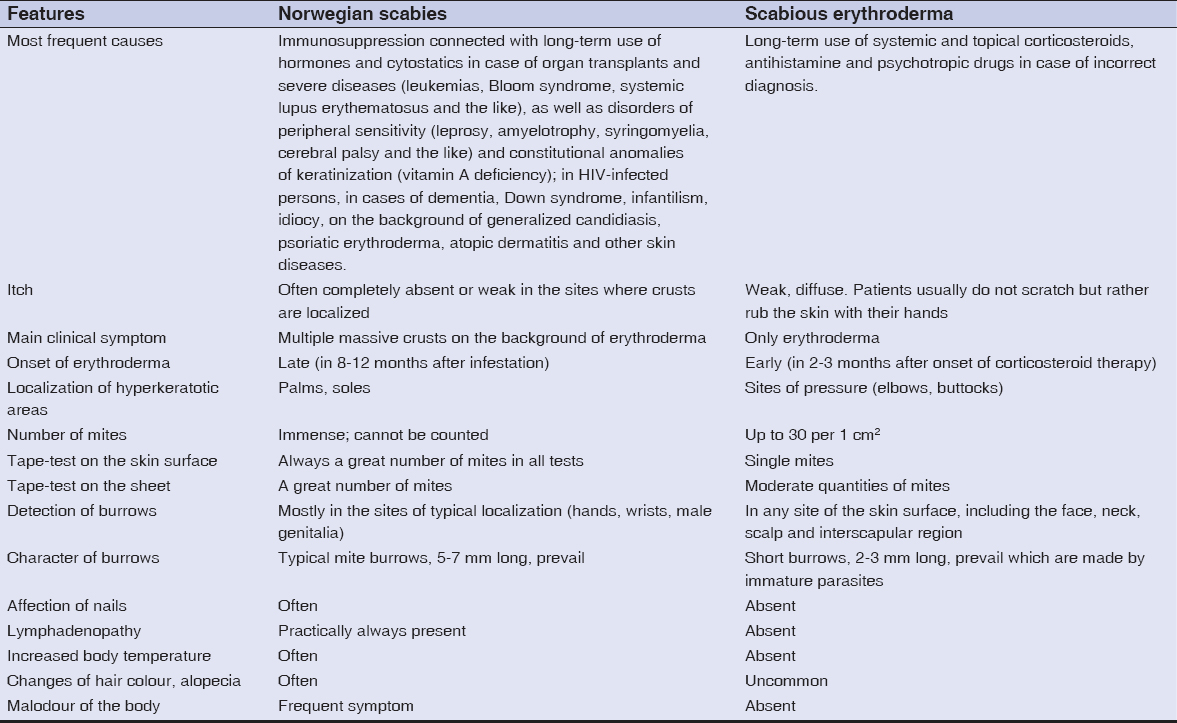
Scabious erythroderma is a rare clinical form of scabies. In fact, this form precedes Norwegian scabies which is mainly characterized by erythroderma and multiple crusts. Since scabious erythroderma has not been previously singled out as a separate form of scabies, many authors regard it as Norwegian scabies taking hyperkeratotic layers for crusts to which the former do not belong. Since the data of medical literature obtained by means of electron microscopy and cultural analyses confirm the colonization of burrows in Norwegian scabies by Staphylococcus aureus, it can be assumed that, in the first stage, this agent acts as a superallergen causing an allergic reaction which reminds reactions in drug eruption, atopic dermatitis (Hill’s erythroderma), psoriasis, and other skin diseases. This reaction is then followed by a severe exudation and massive multilayer crusts are formed, often on the sites where burrows are located. In this case we deal with Norwegian or crusted scabies.
Norwegian or crusted scabies and scabious erythroderma have many features in common:
- Both forms appear on the background of conditions considerably reducing itch, which contributes to a rapid growth of mite colonization of the patient.
- Intense erythroderma is one of the diagnostically relevant criteria in both cases.
- Sites affected are the face, neck and scalp.
- Multiple burrows are present in sites of their typical localization (hands, wrists, feet, elbows, male genitalia).
- On the background of erythroderma follicular papules are present in the areas of apparently normal skin; lenticular papules are found on male genitalia and, in both sexes, in axillar pits, on the abdomen and buttocks; besides, a small number of vesicles are seen on hands and feet.
- Microepidemics arise around such patients: infested are family members, medical personnel and other patients sharing the same ward.
However, there are some significant differences between these two forms of scabies which are listed in Table 3. They are helpful for making differential diagnosis between Norwegian scabies and scabious erythroderma.
The first place in the diagnostic efficacy belongs to dermatoscopy, the second – to microscopy of epidermal scrapings and the third one – to tape-tests. Our experience shows that in patients with common scabies mites are extremely rarely detected by means of tape-tests.
The examination of bed linen used by patients with scabious erythroderma demonstrates high contagiousness of this scabies form. Hence, on admission of such patients to hospital, or while treating them at home, their underwear and bed linen must be daily disinfected.
CONCLUSION
This is the description of a rare clinical form of scabies, scabious erythroderma. It is based on the analysis of the 5 cases of scabies, whose main clinical manifestation is diffuse erythroderma. The diagnostic criteria of scabious erythroderma and differential diagnosis of Norwegian scabies are given. The invasive potential of this form of the disease on the patient and beyond is evaluated for the first time.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent
Informed consent was obtained from all patients for being included in the study.
REFERENCES
1. Adaskevich UP. [Erythroderma. ?onsilium medicum]. Dermatologiya. 2009;2:28-33.
2. Ndiaye M, Ly F, Dioussé P, Diallo M, Diop A, Diatta BA, Niang SO, Kane A, Dieng MT. [The characteristics of severe forms of psoriasis on pigmented skins: A retrospective study of 102 cases in Dakar, Senagal]. Our Dermatol Online. 2017;8:138-42.
3. Akhyani M, Ghodsi ZS, Toosi S, Dabbaghian H. Erythroderma: a clinical study of 97 cases. BMC Dermatol. 2005;9:1-5.
4. Rym BM, Mourad M, Bechir Z, Dalenda E, Faika C, Iadh AM, et al. Erythroderma in adults: a report of 80 cases. Int J Dermatol. 2005;44:731-35.
5. Brzeziński P.: Scabies in soldiers – personal research and historical outline. Lek Wojsk 2009;87:67-72.
6. Chang P, Quijada Ucelo ZM. [Norwegian scabies in an immunocompromised patient]. Our Dermatol Online. 2017;8:484-6.
7. Alsamarai AM, Alobaidi AHA. Scabies in displaced families: Health care problem that need urgent action. Our Dermatol Online. 2017;8:250-4.
8. Fuchs BS, Sapadin ?N, Robert G, Phelps RG, Rudikoff D. Diagnostic dilemma: crusted scabies superimposed on psoriatic erythroderma in a patient with acquired immunodeficiency syndrome. SKINmed: Dermatol Clin. 2007;6:142-44.
9. Freites A. Human T-lymphotropic virus 1 (HTLV-1), strongyloidiasis and scabies. Infections and associations to considerate. Invest. Clin. 2008;49:455-46.
10. Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-47.
11. Buehlmann M, Beltraminelli H, Strub C. Scabies outbreak in an intensive care unit with 1659 exposed individuals-key. Infect Control Hosp Epidemiol. 2009;30:354-60.
12. Rothe M J, Bernstein M L, Grant-Kels JM. Life-threatening erythroderma: diagnosing and treating the “red man”. Clin Dermatol. 2005;23:206-17.
13. Mehta V, Balachandran C, Monga P, Rao R. Norwegian scabies presenting as erythroderma. Ind J Dermatol Vener Leprol. 2009;75:609-10.
14. Mortazavi H, Abedini R, Sadri F. Crusted scabies in a patient with brain astrocytoma: Report of a case. Int J Infect Dis. 2009;22:451.
15. Almond DS, Green CJ, Geurin DM. Lesson of the week Norwegian scabies misdiagnosed as an adverse drug reaction. British Med J. 2000;320:7226-35.
16. Nekhamkin PB, Sarafanova EA, Koryukina EB. [Cases of Norwegian scabies in neuropsychiatric living in boarding schools]. Inter Scien Prac Conf Dermatovenerol Yekaterinburg. 2005;34:1-3.
17. Svecova D, Chmurova N, Pallova A, Babal P. [Norwegian scabies in immunosuppressed patient misdiagnosed as an adverse drug reaction]. Epidemiol Mikrobiol Imunol. 2009;58:121-3.
18. Chosidow O. Scabies and pediculosis. Lancet. 2000;355: 818.
19. Baccouche ?, Sellam J, Guegan S, Aractingi S. Crusted Norwegian scabies, an opportunistic infection, with tocilizumab in rheumatoid arthritis. Joint Bone Spine. 2011;78:402-4.
20. Monari P, Sala R, Calzavara-Pinton P. Norwegian scabies in a healthy woman during oral cyclosporine therapy. Europ J Dermatol. 2007;17:173.
21. Ya FA. [Norwegian scabies]. Vestnik Dermatol Venerol. 1992;4:67-9.
22. Ekmekci TR, Koslu A. Erythrodermic crusted scabies in a young healthy man. Dermatol Online J. 2012;6:23.
23. Judge MR, Kobza-Black A. Crusted scabies in pregnancy. Br J Dermatol. 1995;132:116-19.
24. Baysal V, Yildirim M, Turkman C, Aridogan B, Aydin G. Crusted scabies in a healthy infant. J Eur Acad Dermatol Venereol. 2004;18:188-90.
25. Di Martino Ortiz B, Macchi H, Rebull CV, Re Dominguez ML, Barboza G. Dermatitis herpetiformis: celiac disease of the skin. Report of two cases. Our Dermatol Online. 2018;9:44-7.
26. Wollina U, Gaber B, Mansour R, Langner D, Hansel G, Koch A. Dermatologic Challenges of Health Care for Displaced People. Lessons from a German Emergency Refugee Camp. Our Dermatol Online. 2016;7:136-8.
27. Shricharith S, Anuradha J, Raghavendra R, Pai S. Entodermoscope: A tool to diagnose and monitor pediculosis captitis. Our Dermatol Online. 2015;6:481-2.
28. Sujatha Vijayalekshmi S. Phthiriasis palpebrarum. Our Dermatol Online. 2012;3:355-7.
29. Koudoukpo C, Atadokpèdé F, Salissou L, Adégbidi H, Balloy BC, Akpadjan F, Agbéssi N, Dégboé B, Padonou F. Mixed form of grave scabies on voluntary cosmetic depigmation land: About a case at the Parakou (Benin) University Hospital Center (UHC). Our Dermatol Online. 2017;8(Suppl. 1):32-5.
30. Obaid HM. Home remedies for Pediculus humanus capitis infection among schoolchildren. Our Dermatol Online. 2018;9:131-6.
31. Mebazaa A, Bedday B, Trabelsi S, Denguezli M. Norwegian scabies, a rare diagnosis in Tunisia. Tun Méd. 2006;84: 654.
32. Diabaté A, Kourouma HS, Vagamon B, Gué I, Kaloga M, Aka BR. Skin pathology of the elderly patients: Case of black African. Our Dermatol Online. 2018;9:19-21.
33. Mathisen GE. Editorial response: of mites and men. lessons in scabies for the infectious diseases clinician. Clin Inf Dis. 1998;27:646-48.
34. Shelley WB, Shelley DE, Burmeister V. Staphylococcus aureus colonization of burrows in erythroderma Norwegian scabies: a case study of iatrogenic contagion. J Amer Acad Dermatol. 1988;19:673-78.
35. Bilan P, Colin-Gorski AM, Chapelon E, Sigal ML, Mahé E. [Crusted scabies induced by topical corticosteroids: A case report]. Arch Pediatr. 2015;22:1292-4.
36. Binić I, Humbert P. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J. Korean ?ed. Science. 2010;25:188-91.
37. Patel A, Hogan P, Walder B. Crusted scabies in two immunocompromised children: successful treatment with oral ivermectin. Australasian J Dermatol. 1999;40:37-40.
38. Harman M, Uçmak D, Akkurt ZM, Türkçü G. Hypereosinophilia in erythrodermic psoriasis: superimposed scabies. Cutis. 2014;94:156-9.
39. Haim ?, Grunwald MH, Kapelushnik J, Moser FM. Hypereosinophilia in red scaly infants with scabies. J Ped. 2005:146:712.
40. Goyal A, Balai M, Mittal A, Khare AK, Gupta LK. Pattern of geriatric dermatoses at a Tertiary Care Teaching Hospital of South Rajasthan, India. Our Dermatol Online. 2017;8:237-41.
41. Towersey L, Cunha MX, Feldman CA, Castro CG, Berger TG. Dermoscopy of Norwegian scabies in a patient with acquired immunodeficiency syndrome. An Bras Dermatol. 2010;85:221-3.
42. Abreu Velez AM, Jiménez-Echavarria AM, Howard MS. Immunoreactivity to Meissner corpuscles and dermal nerves in a bullous arthropod bite reaction. Our Dermatol Online. 2017;8:102-3.
43. Katsumata K, Katsumata K. Simple method of detecting Sarcoptes scabiei var hominis mites among bedridden elderly patients suffering from severe scabies infestation using an adhesive-tape. Int Med. 2006;45:857-9.
44. Walter B, Heukelbach J, Fengler G, Worth C. Comparison of dermoscopy, skin scraping, and the adhesive tape test for the diagnosis of scabies in a resource-poor setting. Arch Dermatol. 2011;147:468.
Notes
Source of Support: Nil
Conflict of Interest: None declared.



Comments are closed.