|
Get Citation
|
|
|
Ruiz-Tagle SA, Figueira MM, Vial V, Espinoza-Benavides L, Miteva M. Micronutrients in hair loss. Our Dermatol Online. 2018;9(3):320-328. |
|
|
Download citation file:
|
Micronutrients in hair loss
Susana A. Ruiz-Tagle1, Marcella M. Figueira2, Verónica Vial3, Leonardo Espinoza-Benavides3, Maria Miteva4
1Dermatology Department, Hospital Militar de Santiago, Chile, 2Instituto Professor Fernando Figueira, Recife, Brasil, 3Universidad de los Andes Medical School, Santiago, Chile, 4University of Miami, USA
Corresponding author: Dr. Leonardo Espinoza-Benavides, E-mail: leoespinoza@hotmail.cl
DOI:10.7241/ourd.20183.25
Submission: 12.12.2017; Acceptance: 03.02.2018
ABSTRACT
Alopecia is a common dermatological complaint. Affected patients are often distressed and attempt to arrest the hair loss by taking various over the counter nutritional supplements containing vitamins and minerals. The evidence supporting their efficacy however is limited. Moreover, there are toxicity reports. We reviewed the literature about the normal levels and the daily dietary needs of the most common micronutrients, their role in the hair follicle cycle as well as their use in the hair loss treatment. 4 independent researchers reviewed a total of 119 papers, and 92 articles published in the English language within the last 30 years were included. Telogen effluvium and alopecia areata have been associated with lower iron, zinc and vitamin D levels. Androgenetic alopecia has been associated with lower iron and vitamin D levels. Both lower and increased vitamin A levels can result in telogen effluvium, but lower levels are associated also with hair breakage. Vitamin C insufficiency results in hair shaft abnormality (cork screw hairs). No data exist about hair loss associated with abnormal biotin levels. The role of micronutrients for the hair follicle function is not completely understood. Empiric treatments of hair loss with micronutrients without confirmed deficiencies have not shown utility.
Key words: Alopecia, Supplements, Vitamins, Iron
INTRODUCTION
Hair follicle cells have a high turnover and active metabolism, requiring a good supply of nutrients and energy. A caloric deprivation or deficiency of several macro and micronutrients, such as proteins, minerals, essential fatty acids, and vitamins, can lead to hair loss [1]. Patients with hair loss, particularly with hair shedding are often distressed by their condition and attempt to arrest the shedding taking multivitamins, minerals and herbal products. While considered helpful by patients the consumption of these products may not be supported by evidence [2,3]. Moreover, reports exist of worsening of hair loss as well as liver toxicity [4].
MATERIALS AND METHODS
In order to assess the current evidence about the role of micronutrients for hair loss and hair growth, we reviewed the major database sources PubMed and Medline by using the key words hair, hair loss, alopecia, telogen effluvium and the names of most common vitamins and minerals listed as ingredients in the commercial “hair” and “hair and nails” supplements. We reviewed the literature about the normal levels and the daily dietary needs for optimal hair growth of the most common micronutrients, their role in the hair follicle cycle as well as their use in the hair loss treatment. A total of 119 papers were reviewed by 4 independent researchers, and 92 articles published in English language within the last 30 years were selected for inclusion. All articles were peer-reviewed with available full-text texts in English or Spanish, providing primary data. Also data from the World Health Organization (WHO) and Institute of Medicine (US) about Dietary Reference Intakes and Recommended Dietary Allowance were included.
RESULTS
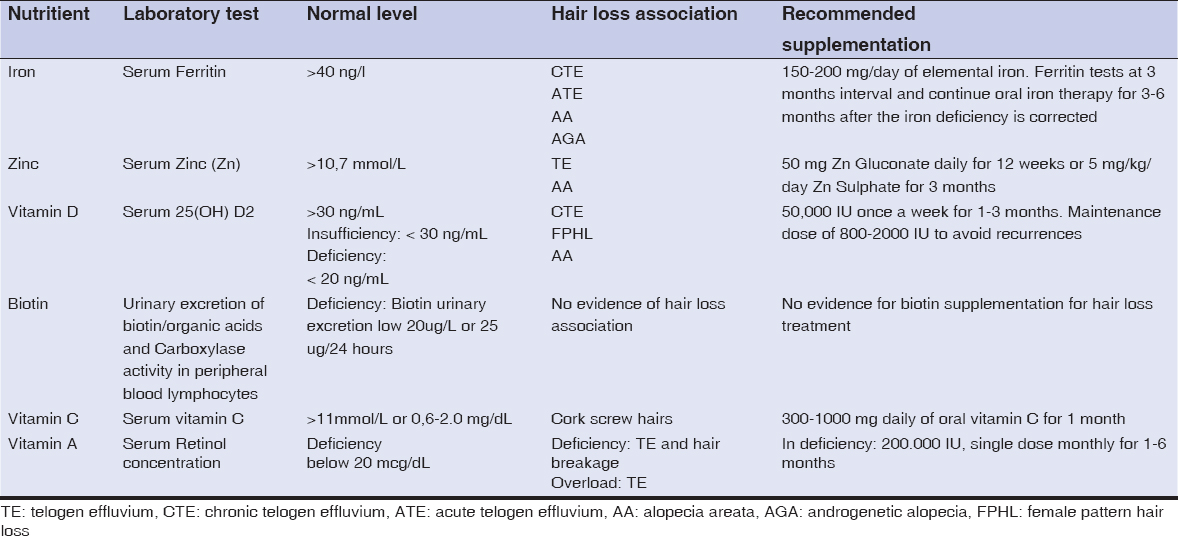
Our results are summarized in Table 1.
1. Iron
Iron participates in the structure of many molecules in the body, such enzymes, cytochromes and transcription factors, and is involved in many critical physiologic processes. It is a catalyst in oxidation-reduction reactions and can control DNA synthesis in dividing cells.
Serum ferritin is the standard test for assessing iron stores because it is one of the most sensitive and specific markers of iron deficiency. It is directly related to intracellular ferritin and total iron reserves [5,6].
Normal levels
The Recommended Dietary Allowance (RDA) for men of any age and for postmenopausal women is 8 mg/day; and for premenopausal women is 18 mg/day.
A cut-off of 41 ng/L of the ferritin’s serum level has sensitivity and specificity of 98% in detecting iron deficiency [7]. Serum ferritin values < 12 ng/l suggest absent iron stores and it is considered diagnostic for iron deficiency anemia [8]. The proposed optimal ferritin level for hair regrowth is 70 ng/L [7,9,10].
It must be considered that ferritin is an acute phase protein, and in neoplasia, infections and inflammatory diseases it may be falsely elevated despite of the low iron reserve. C Reactive Protein levels or erythrocyte sedimentation levels can be used in such cases to rule out false negative results [11].
Causes of deficiency
Iron deficiency is the most common nutritional deficiency in the world. Data from the Third National Health and Nutrition Examination Survey (NHANES III; 1999-2000) indicated that iron deficiency anemia was present in 1 to 2 percent of adults. Iron deficiency without anemia was found in 9-16% of females aged 12-49 years and it was two times higher in non-Hispanic American women. The prevalence of iron deficiency in males aged 16-69 years was 2% [12].
In premenopausal women, the most common causes of iron deficiency anemia are menstrual loss and pregnancy, whereas gastrointestinal blood loss and malabsorption are most common in men and postmenopausal women [6]. Also, borderline iron deficient diets such as vegetarian and vegan diets are another common cause. Good food sources of iron include red meat, egg yolks, green leafy vegetables, lentils, and beans; however, non-animal foods provide less bioavailable-ingested iron. Iron is absorbed mainly in the epithelium of distal duodenum and proximal jejunum [13].
Many patients with iron deficiency and even anemia are asymptomatic. When present, clinical symptoms include hair loss, cheilitis and koilonychia [9].
Iron and hair
Currently, the role of iron on the hair follicle biology is not completely understood and the exact mechanism by which iron deficiency affects hair is also unclear.
It is believed that decreased iron bioavailability may impair the proliferation of the follicular matrix cells. Dividing cells require higher levels of ferritin. An abnormal balance between cellular ferritin and free iron has been suspected as a mechanism for abnormal hair growth [14].
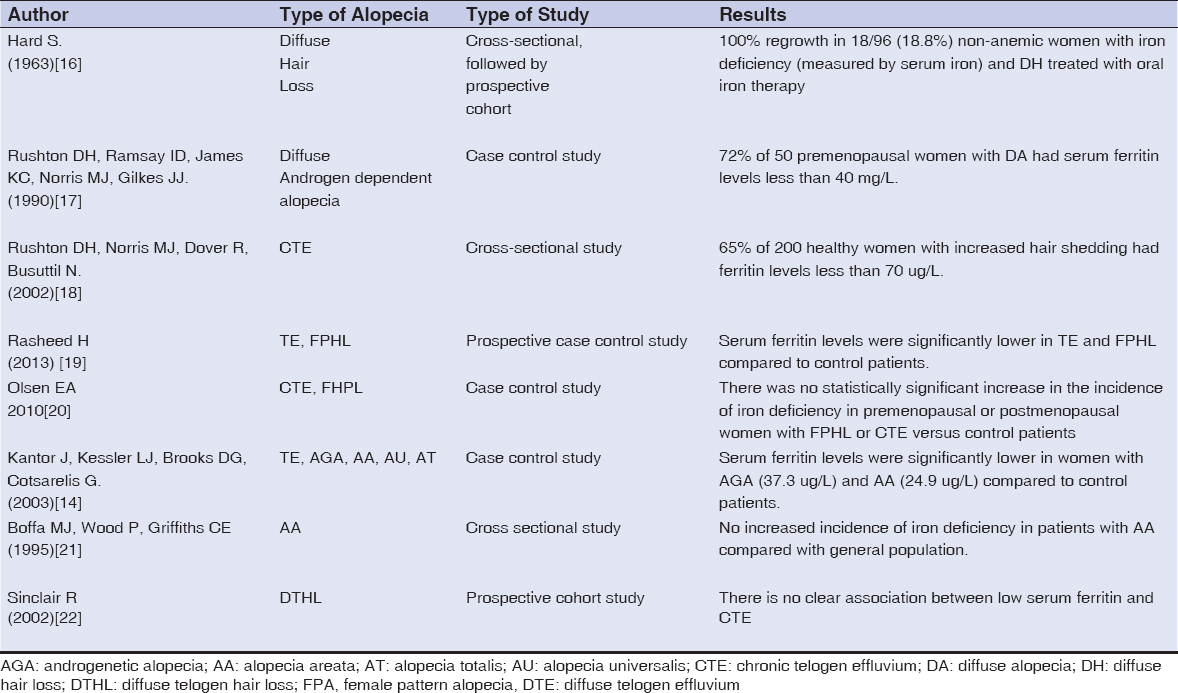
In 2008, Du et al. described iron-dependent genes in the hair follicle bulge whose mutation causes high levels of hepcidin, a liver protein that decreases iron absorption [15]. In 1963, Hard first suggested the role of iron as etiological factor in diffuse hair loss in iron deficient non-anemic women [16]. Since then various studies have evaluated the association; most of them have addressed only women with non-cicatricial alopecia. Data are contradictory and difficult to compare due to discrepancy in the study designs, the variables assessed and the population included (Table 2). Kantor et al proposed the “Threshold hypothesis”, stating that decreased iron stores can lower the threshold to develop different type of alopecia depending on the genetic predisposition and the family history [14].
Treatment recommendation
The recommended oral daily dose for the treatment of iron deficiency in adults is in the range of 150-200 mg/day of elemental iron. It can be given by mouth, under forms of ferrous sulfate, gluconate or fumarate. Better bioavailability of elemental iron per tablet is derived from the fumarates (33% of elemental iron, versus 20% and 12% for sulfate and gluconate respectively) [12], but there is no evidence that one is more effective than the others. Sulfates are worst tolerated as they can cause gastrointestinal upset and constipation. The daily dose can be divided in order to improve the tolerance and absorption.
Concomitant treatment with ascorbic acid, 500-1000 mg per day and L-Lysine 1000 mg per day, may also enhance the absorption [18]. It is recommended to repeat ferritin tests at 3 months interval and continue the oral iron therapy for 3-6 months after the iron deficiency is corrected [6,7,11].
2. Zinc
Zinc is an essential trace mineral that participates in the structure and function of proteins, such as enzymes, transcription factors, hormonal receptor sites, and biologic membranes throughout the body. It is also involved in signal transduction, gene expression, and plays a regulatory role in apoptosis [23]. Zinc is crucial for the proper function of lymphocytes, neutrophils and Natural Killer cells in the immune response, as well as for the skin barrier [24].
Serum or plasma zinc level is the standard test for assessing zinc status [25].
Normal levels
RDA for zinc is 11 mg and 8 mg per day for men and women respectively [26]. The lower limit of normal (morning) fasting plasma zinc has been set at 10.7 mmol/L (700 mg/L) [27].
Causes of deficiency
Severe zinc deficiency has been documented in patients on parenteral feeding without adequate zinc intake and in cases of acrodermatitis enteropathica, an inherited disorder of zinc absorption caused by a mutation in a zinc transporter [28].
Acquired zinc deficiency can also be caused by insufficient uptake from food, intestinal malabsorption syndromes or pregnancy. Long-term alcohol consumption is associated with impaired zinc absorption and increased urinary zinc excretion [21]. Avoidance of red meat by young women can be a cause of concomitant iron and zinc deficiency [29]. The first source of zinc from diet is red meat; other good sources are beans, nuts, crab and lobster. Phytates present in cereals and legumes inhibit the zinc absorption [30].
Cutaneous manifestations present as impaired wound healing and an increased susceptibility to infections, paronychia, periorificial dermatitis, diffuse alopecia [31], and hair color and texture changes [25].
Zinc and hair
The exact role of zinc in the function of the hair follicle is unclear. Zinc has been considered a hair growth modulator and immunomodulator because the DNA polymerase is zinc dependent and zinc acts in multiple aspects of T-lymphocyte activation, signal transduction and cellular apoptosis [24,32]. Zinc deficiency has been related to alopecia areata [33–36], and telogen effluvium [35,37].
Some studies have found lower zinc serum levels in patients with alopecia areata comparing with controls. It has also been shown in alopecia areata that the disease duration, severity and resistance to therapies are correlated inversely with low serum zinc levels [38].
Treatment recommendations
There is scarce evidence on the proper zinc supplementation and the therapeutic response in alopecia areata. Zinc Gluconate dosed as 50 mg daily for 12 weeksproduced regrowth in 15 patients with alopecia areata who had low serum zinc level. Positive therapeutic effects were observed in 9 out of 15 patients (66.7%) although this was not statistically significant [33]. The most recent and the only double blind, cross over study used Zinc Sulphate in a dose of 5 mg/kg/day for 3 months in patients with alopecia areata which resulted in hair regrowth for 60% of the group receiving treatment [34].
Zinc can be supplemented using several forms such as zinc gluconate, zinc sulfate and zinc acetate with different elemental zinc contribution [39]. There are no data about the differences in the efficacy.
3. Vitamin D
Vitamin D is a fat-soluble vitamin belonging to the family of steroid hormones that plays an important role in the calcium homeostasis and musculoskeletal health.
Vitamin D consists of 2 bioequivalent forms, Ergocalciferol (vitamin D2) and Cholecalciferol (vitamin D3). The main source in the body is the endogenous synthesis in the skin as a result of the action of ultraviolet B radiation on 7-dehydrocholesterol, which results in the formation of vitamin D3. The skin is the only organ capable of synthesizing and activating Vitamin D, in addition to expressing its receptor [40].
Vitamin D can be obtained exogenously from few foods like fatty fish, fish liver oil, egg yolk and some mushrooms. Ingested and cutaneous produced vitamin D needs 2 hydroxylation steps, first in the liver, turning into Calcidiol or 25-hydroxyvitamin D, and then in the kidneys to turn into its active metabolite, Calcitriol or 1,25- (OH) 2 D [41].
The most stable form of the vitamin D in the serum is 25 hydroxyvitamin D or 25(OH) D, which is routinely measured to assess the vitamin D status. Besides being the predominant circulating form, it also has a longer half-life [42].
Normal levels
The optimal 25(OH)D serum level is 30 ng/ml (75nmol/L) [43,44]. In 2003, the World Health Organization (WHO) defined vitamin D insufficiency as serum 25(OH) D below 20 ng/ml [45]. Other authors define Vitamin D deficiency as serum 25 (OH) D less than 20 ng/ml and insufficiency below 30 ng/ml [46,47].
Causes of deficiency
Conditions associated with vitamin D deficiency are malnutrition, intestinal malabsorption, especially affecting the proximal small intestine, obesity and some paraneoplasic syndromes [42]. Vitamin D deficiency in healthy adults has been estimated to affect up to 30% of the population [48–50].
Among the risk factors are dark skin, very low sunlight exposure, atmospheric pollution, and multiple within short interval pregnancies, vegetarian diet and some medications such anticonvulsants, rifampicin, antiretroviral agents and corticosteroids [51].
Vitamin D and hair
The action of 1,25- (OH) 2 D is mediated by its binding to the Vitamin D receptor (VDR) which is a member of the nuclear receptor superfamily. VDR distribution on the body is not restricted to organs involved in calcium and bone metabolism but also in the cells of the immune system [42], and in appendageal structures such as the hair follicles [52].
In the hair follicle, the VDR is expressed in the mesodermal dermal papilla cells and the epidermal keratinocytes depending on the stage of the hair cycle. VDR expression in the hair follicle is increased during late anagen and catagen, correlating with proliferation and differentiation of the keratinocytes in preparation for the new hair cycle [53]. Lack of VDR in the keratinocytes as opposed to the dermal papilla would cause its dissociation from hair bulb by the end of catagen, leading to defective initiating of subsequent anagen phase [41,54]. VDR therefore exerts a regulatory role on the hair cycle, independent of the vitamin D binding [55].
Patients with mutations in the VDR, such as hereditary vitamin D-resistant rickets (Vitamin D-dependent rickets type IIA) have normal hair at birth due to the normal hair cycle in the fetus; however, they develop alopecia totalis between 1 to 3 months of age, after the first hair is shed [56,57].
It has been shown among 80 women with chronic telogen effluvium and female pattern of hair loss that the serum vitamin D level was significantly lower compared to controls [18].
A significant lower serum 25 (OH) D level (below 20 ng/ml) were observed in patients with alopecia areata compared with a healthy control group [49, 58–60].
Disease severity in alopecia areata is inversely correlated with the serum levels of vitamin D [49].
Treatment recommendations
People with normal serum level of 25(OH) D are advised to take a supplement containing 800 IU of vitamin D per day to maintain a normal level [40,47].
D2 (ergocalciferol) and D3 (cholecalciferol) are available as dietary supplements. Both seem to be effective in preventing or treating vitamin D deficiency. The longer half-life of D3 suggests that less frequent dosing may be needed. Supplements of vitamins D2 and D3 should be taken with a meal containing fat to ensure maximum absorption [61].
There are no accepted guidelines for treating vitamin D deficiency and insufficiency. A recent review recommends the use of vitamin D3 over vitamin D2 [62]. One time dose of vitamin D3 of at least 300,000 IU is most effective in improving vitamin D status for up of 3 months. However, the most widely used mode of supplementation is an average weekly dose of 50,000 IU (cholecalciferol) for 1-3 months, depending on the severity of Vitamin D deficiency. A maintenance daily dose of 800 to 2000 IU or more will be needekd to avoid recurrent deficiency [40,52,61].
Vitamin D topical analogues have been tested in mice with congenital alopecia with positive response [63]. In human studies, topical calcitriol has shown to prevent alopecia induced by chemotherapy agents (paclitaxel and cyclophosphamide) [56].
4. Biotin
Biotin is an essential nutrient; a water-soluble vitamin classified as a B-complex vitamin. Biotin serves as a coenzyme for carboxylation reactions on fatty acids, aminoacids and glucose metabolism and has an essential role in gene regulation [64,65].
The main source of biotin is the diet; it is widely distributed in foods like egg yolk, cereals and vegetables. Evidence suggests that dietary biotin is 100% bioavailable. It is also synthetized by normal intestinal microflora, but it is unknown how much this source contributes to the biotin status [66].
To achieve its active form and to be absorbed in the intestine, biotin is subjected to a proteolysis. Biotinidase is a critical enzyme in this process. There are hereditary disorders of biotinidase deficiency that can be detected with a newborn screening [66].
Normal levels
There is no conclusive data on validated markers for assessing the biotin status. Measuring urinary excretion of biotin and organic acids such 3- Hydroxyisovaleric and quantifying biotinylated carboxylases in lymphocytes have been utilized. The latter has shown to be the most reliable marker [67]. A low plasma biotin concentration is not a sensitive indicator of inadequate biotin intake.
Deficiency of Biotin has been defined as urinary excretion less than 20ug/L or 25 ug/24 hours [68]. The adequate intake (AI) for biotin is 30 µg/d in men and women [69].
Causes of deficiency
Real biotin deficiency can be observed only in rare and specific conditions: a diet that contains raw egg whites, patients receiving parenteral nutrition without biotin supplementation, and treatments with anticonvulsants such primidone, and carbamazepine [70,71]. Cutaneous findings include severe dermatitis, dry skin, seborrheic dermatitis, fungal infections, macular rash, fine and brittle hair and hair loss [54].
Biotin and hair
There is no evidence regarding direct effect of biotin on the hair follicle development and cycle. There is no data that biotin is related to hair disorders either.
Treatment recommendations
There are no published data supporting the evidence that biotin supplements can be an effective treatment of hair loss.
5. Vitamin C
Vitamin C is a water-soluble vitamin and an essential micronutrient. It is a potent antioxidant and is required for the biosynthesis of collagen, specifically procollagen triple helix and also is needed in the synthesis of catecholamines [72]. It also plays an important role in immune function and modulates iron absorption, transport, and storage [73].
Normal levels
Recommended daily intake in adults is 90 mg in men and 75 mg in women [69].
Measuring the plasma vitamin C levels assesses the vitamin C status. Normal plasma level is in the range of 0,4- 0,9 mg/dL. Vitamin C deficiency is defined as plasma level less than 0,2 mg/dL [74].
Causes of deficiency
According to NAHNES 2003-2004, 7.1% of the total population suffers from vitamin C deficiency, with the smokers being at the most risk. The principal cause of deficiency is the minimal consumption of fruits and vegetables [75]. The clinical presentation of Vitamin C deficiency is scurvy, with skin manifestations due to decreased and altered collagen production [76,77].
Vitamin C and hair
Vitamin C promotes hair shaft elongation in cultured human hair follicles and triggers hair growth in mice by progression from telogen to anagen. This has been achieved by increasing the Insulin Growth Factor 1 (IGF1) production in the dermal papilla cells [78].
Treatment recommendations
The recommended treatment for Vitamin C deficiency is 300-1000 mg daily of oral vitamin C for 1 month [79,80].
6. Vitamin A
Vitamin A is a fat-soluble vitamin. There are two main forms of vitamin A: 1) retinoids or preformed vitamin A and 2) carotenoids or provitamin A. Retinoids are the active form. The common food sources for retinoids are animal derived food (eggs, chicken, fish, and meat). Leafy greens, orange and yellow vegetables and nuts are good sources of carotenoids. Vitamin A has a role in growth, vision, epithelial differentiation, immune function and reproduction. The most common symptom of vitamin A deficiency is xerophthalmia with night blindness [81].
Normal levels
The retinol RDA for adults is 3,000 IU for men and 2,300 IU for women [18]. Serum retinol concentration is the most common method used to evaluate vitamin A status. Other methods such as dose response tests and isotope dilution assays attempt to evaluate liver reserves of vitamin A but are not feasible on daily basis.81 Vitamin A deficiency is defined as retinol serum level below 20µ/dL [83].
Causes of deficiency
Vitamin A deficiency is rare in developed nations but remains a concern in developing countries, particularly in areas with poor nutrition. Several factors such as malnutrition and fat malabsorption can lead to vitamin A deficiency. Hypervitaminosis A is seen with long-term supplementation and oral retinoid treatments [84].
Vitamin A and hair
There is genetic evidence that the alfa retinoid nuclear receptor forms a dimer with Vitamin D receptor and plays a major role in controlling hair cycling [85]. Retinoids play a crucial role for the anagen initiation, and depletion of vitamin A results in epidermal interfollicular hyperplasia with keratinocyte hyperproliferation and aberrant terminal differentiation, accompanied by an inflammatory reaction of the skin [86].
Vitamin A deficiency causes ichthyosis-like skin changes and is often associated with telogen effluvium and fragility of the hair [87,88].
Iatrogenic retinoid-induced hair loss is frequently observed in clinical practice. It has been shown that retinoids can inhibit hair shaft formation during anagen and induce premature catagen [89]. Telogen effluvium can occur with isotretinoin therapy (mostly in doses over 0.5 mg/kg/24 h)[90]. This generally occurs after 3 to 8 weeks of treatment and stops 6 to 8 weeks after stopping it. However, telogen effluvium is more common with acitretin treatment in doses of 25 mg or more daily [91]. Isotretinoin – associated telogen effluvium may also be attributed to an effect on the biotinidase activity [90,92].
Treatment recommendations
In vitamin A deficiency, a single dose of 200,000 IU is given by mouth every 4-6 months [82]. Telogen effluvium in the course of systemic isotretinoin treatment has a benign reversible nature and usually requires no treatment.
CONCLUSION
Our results show that the role of micronutrients for the hair follicle function and the mechanisms by which deficiency could lead to hair loss are not completely understood. Empiric treatments of hair loss conditions without confirmed deficiencies have not shown utility.
REFERENCES
1. Finner AM. Nutrition and hair:deficiencies and supplements. Dermatol Clin. 2013;31:167–7.
2. Reuter J, Merfort I, Schempp CM. Botanicals in dermatology:an evidence-based review. Am J Clin Dermatol. 2010;11:247–67.
3. Bandaranayake I, Mirmirani P. Hair loss remedies–separating fact from fiction. Cutis. 2004;73:107–14.
4. Fernández J, Navascués C, Albines G, Franco L, Pipa M, Rodríguez M. Three cases of liver toxicity with a dietary supplement intended to stop hair loss. Rev Esp Enfermedades Dig Organo Soc Esp Patol Dig. 2014;106:552–5.
5. Beutler E, Felitti V, Ho NJ, Gelbart T. Relationship of body iron stores to levels of serum ferritin, serum iron, unsaturated iron binding capacity and transferrin saturation in patients with iron storage disease. Acta Haematol. 2002;107:145–9.
6. Goddard AF, James MW, McIntyre AS, Scott BB, British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309–16.
7. St Pierre SA, Vercellotti GM, Donovan JC, Hordinsky MK. Iron deficiency and diffuse nonscarring scalp alopecia in women:more pieces to the puzzle. J Am Acad Dermatol. 2010;63:1070–6.
8. Thomas DW, Hinchliffe RF, Briggs C, Macdougall IC, Littlewood T, Cavill I, et al. Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol. 2013;161:639–48.
9. Trost LB, Bergfeld WF, Calogeras E. The diagnosis and treatment of iron deficiency and its potential relationship to hair loss. J Am Acad Dermatol. 2006;54:824–44.
10. Rushton DH, Dover R, Norris MJ, Gilkes JJH. Iron and hair loss in women;what is deficiency? This is the real question!. J Am Acad Dermatol. 2007;56:518–9;author reply 519.
11. Elston DM. Commentary:Iron deficiency and hair loss:problems with measurement of iron. J Am Acad Dermatol. 2010;63:1077–82.
12. Centers for Disease Control and Prevention. Iron deficiency-US, 1999-2000 Morb Mortal Wkly Rep. 2002;51:897–9.
13. Gulec S, Anderson GJ, Collins JF. Mechanistic and regulatory aspects of intestinal iron absorption. Am J Physiol Gastrointest Liver Physiol. 2014;307:G397–409.
14. Kantor J, Kessler LJ, Brooks DG, Cotsarelis G. Decreased serum ferritin is associated with alopecia in women. J Invest Dermatol. 2003;121:985–8.
15. Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–92.
16. Hård S. Non-anemic iron deficiency as an etiological factor in diffuse loss of hair of the scalp in women. Acta Derm Venereo. 1963;43:562-9
17. Rushton DH, Ramsay ID, James KC, Norris MJ, Gilkes JJ. Biochemical and trichological characterization of diffuse alopecia in women. Br J Dermatol. 1990;123:187–97.
18. Rasheed H, Mahgoub D, Hegazy R, El-Komy M, Abdel Hay R, Hamid MA, et al. Serum ferritin and vitamin d in female hair loss:do they play a role?. Skin Pharmacol Physiol. 2013;26:101–7.
19. Rushton DH, Norris MJ, Dover R, Busuttil N. Causes of hair loss and the developments in hair rejuvenation. Int J Cosmet Sci. 2002;24:17–23.
20. Olsen EA, Reed KB, Cacchio PB, Caudill L. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991–9.
21. Boffa MJ, Wood P, Griffiths CE. Iron status of patients with alopecia areata. Br J Dermatol. 1995;132:662–4.
22. Sinclair R. There is no clear association between low serum ferritin and chronic diffuse telogen hair loss. Br J Dermatol. 2002;147:982–4.
23. Hambidge KM, Krebs NF. Zinc deficiency:a special challenge. J Nutr. 2007;137:1101–5.
24. Shankar AH, Prasad AS. Zinc and immune function:the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68(2 Suppl):447S-63S.
25. Maverakis E, Fung MA, Lynch PJ, Draznin M, Michael DJ, Ruben B, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116–24.
26. Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc [Internet]. Washington (DC):National Academies Press (US);2001 [cited 2015 Oct 15]. Available from:http://www.ncbi.nlm.nih.gov/books/NBK222310
27. Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol Organ Soc Miner Trace Elem GMS. 2006;20:3–18.
28. Küry S, Dréno B, Bézieau S, Giraudet S, Kharfi M, Kamoun R, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:239–40.
29. Hunt JR. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr. 2003;78(3 Suppl):633S-9S.
30. Organization WH, Nutrition WEC on TE in H. Trace elements in human nutrition?:report of a WHO expert committee [meeting held in Geneva from 9 to 17 April 1973]. 1973 [cited 2015 Dec 29];Available from:http://www.who.int/iris/handle/10665/41057
31. Kumar P, Lal NR, Mondal AK, Mondal A, Gharami RC, Maiti A. Zinc and skin:a brief summary. Dermatol Online J. 2012;18:1.
32. Plonka PM, Handjiski B, Popik M, Michalczyk D, Paus R. Zinc as an ambivalent but potent modulator of murine hair growth in vivo- preliminary observations. Exp Dermatol. 2005;14:844–53.
33. Park H, Kim CW, Kim SS, Park CW. The therapeutic effect and the changed serum zinc level after zinc supplementation in alopecia areata patients who had a low serum zinc level. Ann Dermatol. 2009;21:142–6.
34. Sharquie KE, Noaimi AA, Shwail ER. Oral zinc sulphate in treatment of alopecia areata. J Clin Exp Dermatol Res. 2012;3:150
35. Kil MS, Kim CW, Kim SS. Analysis of serum zinc and copper concentrations in hair loss. Ann Dermatol. 2013;25:405–9.
36. Bhat YJ, Manzoor S, Khan AR, Qayoom S. Trace element levels in alopecia areata. Indian J Dermatol Venereol Leprol. 2009;75:29–31.
37. Abdel Aziz AM, Sh Hamed S, Gaballah MA. Possible Relationship between Chronic Telogen Effluvium and Changes in Lead, Cadmium, Zinc, and Iron Total Blood Levels in Females:A Case-Control Study. Int J Trichology. 2015;7:100–6.
38. Abdel Fattah NSA, Atef MM, Al-Qaradaghi SMQ. Evaluation of serum zinc level in patients with newly diagnosed and resistant alopecia areata. Int J Dermatol. 2016;55:24-9.
39. Corbo MD, Lam J. Zinc deficiency and its management in the pediatric population:a literature review and proposed etiologic classification. J Am Acad Dermatol. 2013;69:616–24.e1.
40. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D [Internet]. Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Washington (DC):National Academies Press (US);2011 [cited 2015 Oct 15]. Available from:http://www.ncbi.nlm.nih.gov/books/NBK56070
41. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health:lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76.
42. Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91:115–24.
43. Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–50.
44. Weinstock MA, Moses AM. Skin cancer meets vitamin D:the way forward for dermatology and public health. J Am Acad Dermatol. 2009;61:720–4.
45. WHO Scientific Group on the Prevention and Management of Osteoporosis (2000?:Geneva S. Prevention and management of osteoporosis?:report of a WHO scientific group. 2003 [cited 2015 Oct 15];Available from:http://www.who.int/iris/handle/10665/42841
46. Gallagher JC, Sai AJ. Vitamin D insufficiency, deficiency, and bone health. J Clin Endocrinol Metab. 2010;95:2630–3.
47. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
48. Mitchell DM, Henao MP, Finkelstein JS, Burnett-Bowie S-AM. Prevalence and predictors of vitamin D deficiency in healthy adults. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2012;18:914–23.
49. Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299–304.
50. Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S-64S.
51. Pearce SHS, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 2010;340:b5664.
52. Płudowski P, Karczmarewicz E, Bayer M, Carter G, Chlebna-SokółD, Czech-Kowalska J, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe – recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol. 2013;64:319–27.
53. Demay MB. The hair cycle and Vitamin D receptor. Arch Biochem Biophys. 2012;523:19–21.
54. Demay MB, MacDonald PN, Skorija K, Dowd DR, Cianferotti L, Cox M. Role of the vitamin D receptor in hair follicle biology. J Steroid Biochem Mol Biol. 2007;103:344–6.
55. Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011;347:80–9.
56. Amor KT, Rashid RM, Mirmirani P. Does D matter? The role of vitamin D in hair disorders and hair follicle cycling. Dermatol Online J. 2010;16:3.
57. Bergman R, Schein-Goldshmid R, Hochberg Z, Ben-Izhak O, Sprecher E. The alopecias associated with vitamin D-dependent rickets type IIA and with hairless gene mutations:a comparative clinical, histologic, and immunohistochemical study. Arch Dermatol. 2005;141:343–51.
58. Yilmaz N, Serarslan G, Gocke C. Vitamin D concentrations are decreased in patients with alopecia areata. Vitam Trace Elem. 2012;1:105–9.
59. Mahamid M, Abu-Elhija O, Samamra M, Mahamid A, Nseir W. Association between vitamin D levels and alopecia areata. Isr Med Assoc J IMAJ. 2014;16:367–70.
60. D’Ovidio R, Vessio M, d’Ovidio FD. Reduced level of 25-hydroxyvitamin D in chronic/relapsing Alopecia Areata. Dermatoendocrinol. 2013;5:271–3.
61. Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults:when to test and how to treat. Mayo Clin Proc. 2010;85:752–7;quiz 757–8.
62. Kearns MD, Alvarez JA, Tangpricha V. Large, single-dose, oral vitamin d supplementation in adult populations:a systematic review. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2014;20:341–51.
63. Vegesna V, O’Kelly J, Uskokovic M, Said J, Lemp N, Saitoh T, et al. Vitamin D3 analogs stimulate hair growth in nude mice. Endocrinology. 2002;143:4389–96.
64. Said HM. Biotin:the forgotten vitamin. Am J Clin Nutr. 2002;75:179–80.
65. Fernandez-Mejia C, Lazo-de-la-Vega-Monroy M-L. Biological Effects of Pharmacological Concentrations of Biotin. J Evid-Based Complement Altern Med. 2011;16:40–8.
66. Zempleni J, Kuroishi T. Biotin. Adv Nutr Bethesda Md. 2012;3:213–4.
67. Eng WK, Giraud D, Schlegel VL, Wang D, Lee BH, Zempleni J. Identification and assessment of markers of biotin status in healthy adults. Br J Nutr. 2013;110:321–9.
68. Sardesai V. Introduction to Clinical Nutrition, Third Edition. CRC Press;2011. 706 p.
69. Food and Nutrition Board, Institute of Medicine. Biotin. Dietary Reference Intakes:Thiamin, Riboflavin, Niacin, Vitamin B6, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC:National Academy Press;1998;374–89.
70. Kurowski HL, Gospe SM, Zeman FJ, Grivetti LE. Nutritional factors and anticonvulsant therapies:effect on growth in children with epilepsy. Am J Clin Nutr. 1993;58:858–61.
71. Said HM, Redha R, Nylander W. Biotin transport in the human intestine:inhibition by anticonvulsant drugs. Am J Clin Nutr. 1989;49:127–31.
72. Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137:2171–84.
73. Gershoff SN. Vitamin C (ascorbic acid):new roles, new requirements?. Nutr Rev. 1993;51:313–26.
74. Loria CM, Whelton PK, Caulfield LE, Szklo M, Klag MJ. Agreement among indicators of vitamin C status. Am J Epidemiol. 1998;147:587–96.
75. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States:2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252–63.
76. Basavaraj KH, Seemanthini C, Rashmi R. Diet in dermatology:present perspectives. Indian J Dermatol. 2010;55:205–10.
77. Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895–906;quiz 907–10.
78. Kwack MH, Shin SH, Kim SR, Im SU, Han IS, Kim MK, et al. l-Ascorbic acid 2-phosphate promotes elongation of hair shafts via the secretion of insulin-like growth factor-1 from dermal papilla cells through phosphatidylinositol 3-kinase. Br J Dermatol. 2009;160:1157–62.
79. Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away:scurvy in the year 2000. Pediatrics. 2001;108:E55.
80. Olmedo JM, Yiannias JA, Windgassen EB, Gornet MK. Scurvy:a disease almost forgotten. Int J Dermatol. 2006;45:909–13.
81. Tanumihardjo SA. Biomarkers of vitamin A status:what do they mean? In:World Health Organization. Report:Priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15–17 September 2010. Geneva, World Health Organization, 2012.
82. World Health Organization. Vitamin A supplements:a guide to their use in the treatment and prevention of vitamin A deficiency and xerophthalmia, 2nd Edition (1997). Available at:http://www.who.int/nutrition/publications/micronutrients/vitamin_a_deficiency/9241545062/en/(Accessed on April 07, 2015)
83. De Pee S, Dary O. Biochemical indicators of vitamin A deficiency:serum retinol and serum retinol binding protein. J Nutr. 2002 Sep;132(9 Suppl):2895S-901S.
84. Myhre AM, Carlsen MH, BMH, SK, Wold HL, Laake P, Blomhoff R. Water-miscible, emulsified, and solid forms of retinol supplements are more toxic than oil-based preparations. Am J Clin Nutr. 2003;78:1152–9.
85. Haussler MR, Haussler CA, Jurutka PW, Thompson PD, Hsieh JC, Remus LS, et al. The vitamin D hormone and its nuclear receptor:molecular actions and disease states. J Endocrinol. 1997;154 Suppl:S57–73.
86. Li M, Chiba H, Warot X, Messaddeq N, Gérard C, Chambon P, et al. RXR-alpha ablation in skin keratinocytes results in alopecia and epidermal alterations. Dev Camb Engl. 2001;128:675–88.
87. Bleasel NR, Stapleton KM, Lee MS, Sullivan J. Vitamin A deficiency phrynoderma:due to malabsorption and inadequate diet. J Am Acad Dermatol. 1999;41(2 Pt 2):322–4.
88. Girard C, Dereure O, Blatière V, Guillot B, Bessis D. Vitamin a deficiency phrynoderma associated with chronic giardiasis. Pediatr Dermatol. 2006;23:346–9.
89. Foitzik K, Spexard T, Nakamura M, Halsner U, Paus R. Towards dissecting the pathogenesis of retinoid-induced hair loss:all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta2 in the dermal papilla. J Invest Dermatol. 2005;124:1119–26.
90. Famenini S, Goh C. Evidence for supplemental treatments in androgenetic alopecia. J Drugs Dermatol JDD. 2014;13:809–12.
91. Katz HI, Waalen J, Leach EE. Acitretin in psoriasis:an overview of adverse effects. J Am Acad Dermatol. 1999;41(3 Pt 2):S7–12.
92. Schulpis KH, Georgala S, Papakonstantinou ED, Michas T, Karikas GA. The effect of isotretinoin on biotinidase activity. Skin Pharmacol Appl Skin Physiol. 1999;12:28–33.
Notes
Source of Support: Nil
Conflict of Interest: None declared.
Comments are closed.