Therapeutic potential of d-δ-tocotrienol rich fraction on excisional skin wounds in diabetic rats
Bijo Elsy1, Aijaz Ahmed Khan1, Veena Maheshwari2
1Department of Anatomy, JN Medical College, Aligarh Muslim University, Aligarh, India; 2Department of Pathology, JN Medical College, Aligarh Muslim University, Aligarh, India
Corresponding author: Bijo Elsy, E-mail: bijobaby22@yahoo.com
Submission: 23.12.2016; Acceptance: 04.03.2017
DOI: 10.7241/ourd.20174.109
ABSTRACT
Introduction: Long-standing hyperglycemia in addition to many of its associated complications also hampers normal wound healing which may be further aggravated in the presence of infection and oxidative stress. Therefore, antioxidant supplementation appears to be strategically relevant for wound healing. This study is designed to explore the therapeutic potential of d-δ-tocotrienol rich fraction (d-δ-TRF) on skin wound healing in both healthy and diabetic rats.
Materials and Methods: Diabetes was induced through single subcutaneous injection of alloxan at the dose of 100 mg/kg at hip region. 24 albino rats were divided into four groups; healthy control, diabetic control, healthy treated and diabetic treated. d-δ-TRF was administered to treated groups (200 mg/kg), orally, daily for 3 weeks. Full thickness excisional skin wounds were. Wound area was studied by assessing the morphological, histomorphological and histological features at weekly intervals and biochemical analyses were performed at the end of 3rd week.
Results: The findings of present study revealed that d-δ-TRF accelerated the skin wound healing by means of early regeneration of both epidermal and dermal components; enhancement of serum protein synthesis, improvement of antioxidant status, maintenance of glycemic condition and controlling serum creatinine levels in diabetic rats.
Conclusion: It is concluded that d-δ-TRF has significant therapeutic potency on the healing of skin wounds in both healthy and diabetics.
Key words: Antioxidant; d-δ-Tocotrienol; Diabetes; Rat; Skin; Wounds
INTRODUCTION
In diabetes wound healing is delayed due to hyperglycemia, infections and oxidative stress [1]. Hyperglycemia is known to causes increased production of free radicals and insufficiencies in the antioxidant system [2]. Excess reactive oxygen species (ROS) is secreted in the inflammatory phase of wound healing by neutrophils and macrophages [3]. Both non-enzymatic antioxidants (e.g., glutathione, vitamin C, vitamin E) and enzymatic antioxidants (e.g., SOD, GPX, PRDX, and catalase) are involved in the fine tuning of ROS level [4]. And an optimal ROS level is distinctive for each step of wound healing [5].
The antioxidants have ability to reduce the diabetes complications by arresting free radical-induced damage [6]. Vitamin E has both saturated (tocopherols) and unsaturated (tocotrienols) forms and is an effective antioxidant. Free-radical scavenging effects of tocotrienols appear superior because of their better distribution in the fatty layers of the cell membrane [7].
Tocotrienols are believed to possess antioxidant, antidiabetic, anti-inflammatory, anticancer, immunostimulating, cardioprotective, neuroprotective, hepatoprotective and nephroprotective properties [8]. Interestingly, the antitumor activity of tocotrienols is not dependent on its antioxidant activity [9,10]. The highly biopotent γ and δ- tocotrienols may play a physiological role in modulating normal mammary gland growth, function and remodeling. Nevertheless anticancer effects on mammary tumor cells by applying these compounds did not display any adverse effect on normal mammary epithelial cell growth [11–13].
The tocotrienol-rich fraction (TRF) of palm oil consists of 25% α -tocopherol and 75% tocotrienol [14]. The concentrations of different constituents of palm oil-derived-TRF per gram are α-tocopherol at 171.1 mg, α-tocotrienol at 190.4 mg, β-tocotrienol 36.0 mg, γ-tocotrienol 211.2 mg and δ-tocotrienol 150 mg [15]. Therefore, TRF being an excellent antioxidant has been effectively used as a nutritional supplement due to its potential therapeutic benefits [16].
In deep partial-thickness burn wounds, the TRF treatment has been shown to accelerate the wound contraction rate, enhance the reepithelialization, the regeneration process and stimulate the granulation tissue formation [14]. According to Musalmah et al [17], supplementation of TRF at 200 mg/kg was able to improved wound healing in type 1 induced diabetic rat.
Data related to the effects of specific tocotrienol isoforms treatment on skin wound healing are scarce. The available very limited studies mainly focused on wound healing effects of TRF [14,17]. Since TRF contain different vitamin E isoforms, it was not possible to determine which isoforms was specifically responsible to promotes skin wound healing. Hence the present study is focused to assess the therapeutic antioxidant potency of d-δ-TRF on full thickness excisional skin wound healing in healthy and diabetic rats by using histological, histomorphological and biochemical parameters.
MATERIALS AND METHODS
Twenty four albino rats of either sex each weighing 230-320g were obtained from central animal house of JN medical college, AMU, Aligarh. The study was approved by Institutional Animal Ethical Committee (No. 8937/2014). Prior to commencement of the experiments, animals were acclimatized to the new environmental condition for a period of one week. They were kept in a well ventilated room and maintained on a standard pellet diet and water [18].
Induction of Diabetes
Diabetes was induced to the diabetic group after deprivation of food for 4 hours, followed by single subcutaneous injection (hip region) of alloxan (100 mg/kg; Alloxan monohydrate from Sigma-Aldrich). Food and water were provided after one hour of injection. Blood was obtained via tail vein for monitoring sugar level by using Glucometer (Dr Morepen gluco one) on the 4th day of alloxan injection. Animals with blood sugar level at 250 mg/dl and above were selected as diabetic for this study. Weight and blood sugar levels of all animals in each group were monitored at weekly intervals [18].
Experimental Groups, Route and Dosage of Treatment
Animals were divided into four groups having 6 rats in each group: (1) healthy control- HC; (2) diabetic control- DC; (3) healthy d-δ-TRF treated- HTT and (4) diabetic d-δ-TRF treated – DTT (200 mg/kg body weight, orally, daily for 3 weeks. Unique E Tocotrienol, tocopherol free, 90% δ and 10% γ tocotrienols, AC Grace Company, P.O Box 570, Big Sandy, TX 75755, USA). Orally supplemented tocotrienol was rapidly taken up by the skin [19]. Dosage of d-δ- tocotrienol rich fraction (200 mg/kg body weight) was based on previous studies of TRF [15,17,20].
Surgical Procedure
All animals received general anesthesia via inhalation of ether and after that, the dorsal surface of thoracic region was shaved and antisepsis was performed over the shaved area. Full thickness excisional skin wounds of 8.5 ± 0.48 mm diameter (an area equivalent to 46.74 ± 0.32 mm2) was made on pinched skin fold of shaved area. Type and size of wound model were very akin to the murine excisional wound model described earlier [21]. Povidone-iodine solution (antisepsis) was applied on the wound and 0.5 ml Voveran (analgesic) and 2 mg single shot of Gentamycin (antibiotic) were also injected simultaneously [18].
Sample Collection and Fixation of Tissue
On completion of 3 weeks animals were sacrificed under deep ether anesthesia and then excised the healed parts of skin with adjacent area. The excised tissues were immersion-fixed in 10% neutral buffered formalin. Blood samples were collected into sterilized vials by direct puncture of heart at the time of sacrifice. Samples were allowed to clot, centrifuged at 2500 rpm for 30 min, the serum was separated and stored in vials and used to assay all biochemical parameters [18].
Macroscopic Examination
The macroscopic changes in the wound healing sequence of events were observed and recorded photographically on 1st, 7th, 14th & 21st day of creation of wounds.
Histopathology & Histomorphometry
Fixed tissue samples were processed for light microscopical studies. The 5 μm thick sections were stained with Haematoxylin & Eosin (H & E), Masson’s Trichrome (MT), Aldehyde Fuchsin with Fast Green (AF with FG) and PicroSirus Red with Fast Green (PSR with FG).
Histomorphometry was performed on both H & E and MT stained sections. While H & E sections were used for measuring the Global Healing Index (GHI), MT stained sections were used for estimation of Global Remodeling Index (GRI). Histological features under x 4 objective lens of trinocular microscope (Olympus, BX40; Japan) were recorded by digital camera (Sony 18.2 MP, Japan) and measurements were made by using software Motic image version 2.0. Measurements related to epidermal thickness and calculation of healing indices were based on the mathematical model for healing and remodeling matrix [22].
Biochemical Estimation & Analysis
- All lipid profiles, serum creatinine and serum total protein content were carried out by using Avantor Benesphera™ clinical chemistry Analyzer C61.
- Enzymatic antioxidant
Serum catalase was assayed by colorimetery as described [23]. The light absorbance of the sample was determined at 620 nm.
- Non-invasive biomarker (oxidative stress parameter)
Serum total antioxidant capacity (TAC) was evaluated using ferric reducing antioxidant power (FRAP) assay [24]. The absorbance of sample was measured at 620 nm using photo colorimeter.
Statistical Analysis
All the data were statistically evaluated and the significance calculated using one way ‘ANOVA’ followed by Tukeys test. All the results were expressed as mean ± SD and P < 0.05 was considered as statistically significant. Student t test was used for comparing the blood sugar level in DTT group before and after supplementation of d-δ-TRF (P < 0.0001).
RESULT
Body weight and Blood Sugar Level
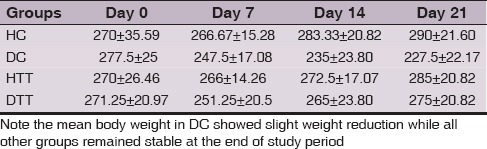
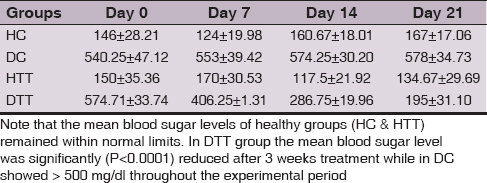
Weight and blood sugar levels of all animals in each group were monitored at weekly intervals. Mean body weight in DC showed slight weight reduction whereas in all other groups (HC, HTT& DTT) remained stable at the end of study period (Table 1). Mean blood sugar levels of healthy groups (HC & HTT) remained within normal limits. In DTT the mean blood sugar level was significantly (P<0.0001) reduced after 3 weeks supplementation of d-δ-TRF while in DC showed > 500 mg/dl throughout the experimental period (Table 2).
Macroscopic Observations
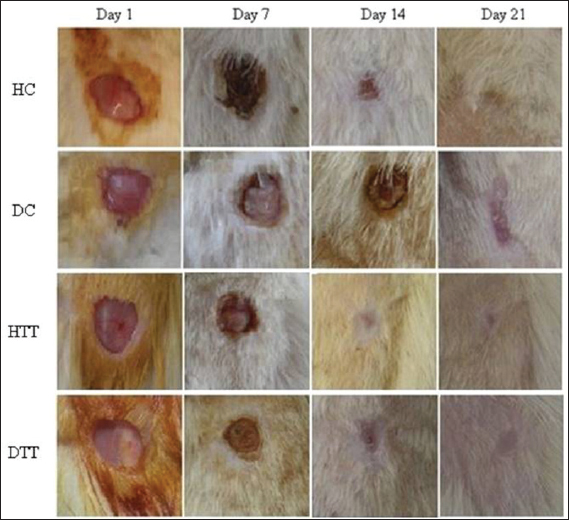
In treated groups remarkable progressive changes in the size of wound area were observed at the end of 14th day compared to control groups (Fig. 1).
Microscopic Observations
Histomorphometry
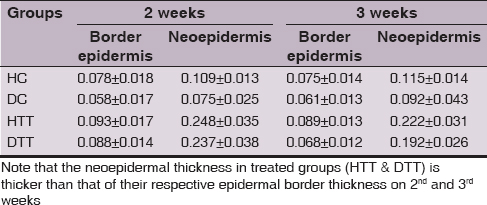
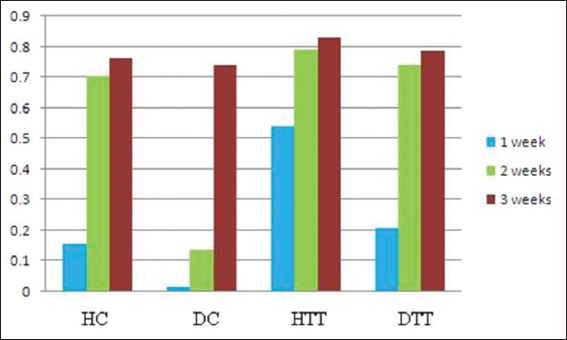
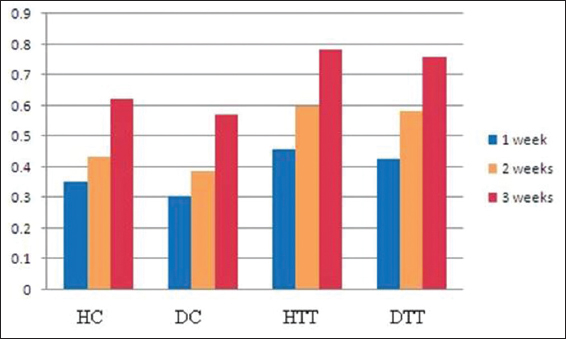
In all groups the neoepidermis was developed at the end of 2nd week. However, in treated groups the mean values of neoepidermis were significantly thicker (P<0.01) than the corresponding epidermal border thickness on 2nd and 3rd weeks (Table 3). During the study period in treated groups the GHI and GRI were significantly higher (P<0.01) compared to control groups (Figs 2 and 3).
Reepithelialization
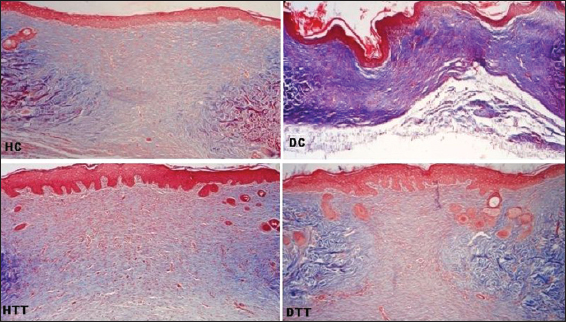
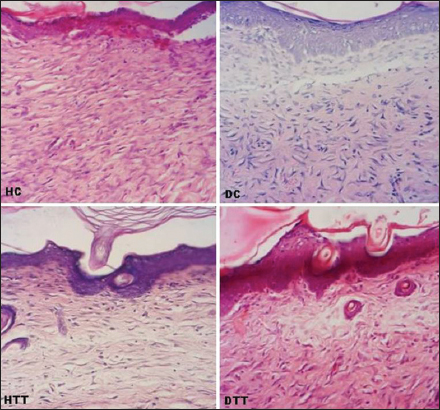
Complete reepithelialization was noticed in all the groups (controls and treated). On 2nd and 3rd weeks, in treated groups well defined interdigitations at dermoepidermal junction appeared on the entire length of neoepidermis (Figs 4, 5a and 7). On 2nd weeks in HC poorly defined interdigitations at wound margins while in DC absence of interdigitations (Fig. 4). On 3rd weeks in control groups these features were restricted to only at the wound margins (Fig. 5a and 7).
Cellular components
At the end of study period, the granulation tissue consists of mainly fibroblasts in all groups. The fibroblasts appeared oval or spindle shaped and scattered in HC. In DC these cells were mainly stellate whereas in treated groups spindle shaped cells lie parallel to the neoepidermis. More cellularity was observed in control groups as compared to treated groups (Fig. 6).
Neovascularization
In treated groups, well formed vertically oriented blood capillaries appeared by the end of 2nd week while they appeared late in HC by the end 3rd week. Swollen capillaries and extravasation of blood cells were seen in DC granulation tissue on 3rd weeks whereas in treated groups less number of vessels was observed on 3rd weeks (Figs 5a and 5b).
Matrix remodeling and Skin appendages
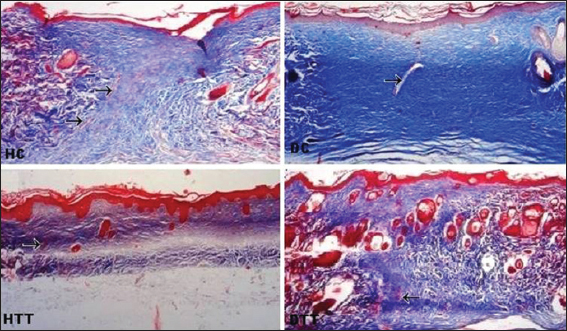
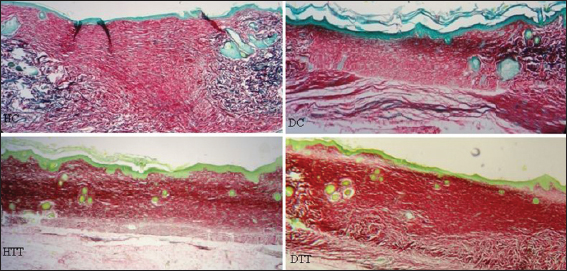
On 2nd and 3rd weeks, in treated groups the collagen fibres in the regenerated dermis were mostly horizontally arranged and compactly interwoven but these fibres were more obliquely placed in HC on 3rd weeks. In DC on 2nd weeks collagen fibres were arranged as wavy pattern and on 3rd weeks these fibres were poorly interlaced in the suprahypodermal area (Figs 4, 5a and 7).
The elastin fibres in control groups were found in the wound margins while in the treated groups these fibres were noticed in one step advanced stage and newly formed smaller fibrils were diffusely arranged in the regenerating dermis on 3rd weeks (Fig. 8).
On 2nd weeks, in control groups the hair follicles were confined to wound margins while in treated groups hair follicles were notice almost in the central part of the wound (Fig. 4). At the end of study period, in treated groups hair follicles and sebaceous glands were in advance stage into the regenerating dermis and newly formed hairs found within the hair follicles and neoepidermal surface. In control groups hair follicles and sebaceous glands remained only at the wound margins (Figs 6 and 7).
Biochemical Analysis
Lipid profiles
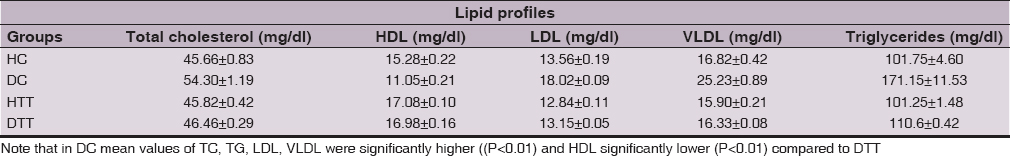
Total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL) and very low density lipoprotein (VLDL) in DC were significantly higher ((P<0.01) compared to DTT. Whereas high density lipoprotein (HDL) in DC showed significantly lower (P<0.01) compared to DTT (Table 4).
Serum creatinine level and serum total protein content
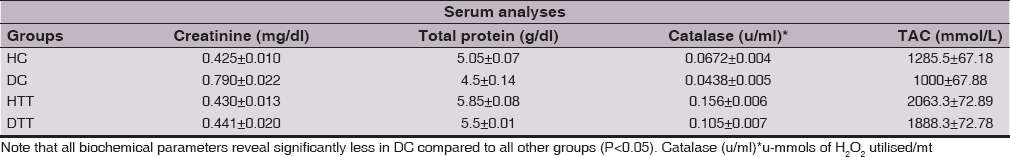
Serum creatinine level in DC were significantly higher ((P<0.01) compared to all other groups. Serum total protein content in treated groups (HTT & DTT) showed significantly higher (P<0.01) compared to control groups (HC & DC) (Table 5).
Enzymatic antioxidant and oxidative stress parameter
Serum catalase activity and total antioxidant capacity in treated groups (HTT & DTT) exhibited significantly higher (P<0.01, P<0.05) compared to control groups (HC & DC). These analyses values in DC showed significantly lower (P<0.05) compared to HC group (Table 5).
DISCUSSION
Impaired wound healing is a well-documented phenomenon in both experimental and clinical diabetes [25]. Free radicals impair the normal wound healing by damaging keratinocyte, endothelial cells, capillary permeability and collagen metabolism [26]. Oxidative stress induces cellular dysfunction and retards angiogenesis and the healing process [27]. Thus, elimination of ROS is an important strategy to improve the healing of wounds in diabetes mellitus patients [28]. The unsaturated isoforms of vitamin E e.g., tocotrienols possess excellent antioxidant activity and suppress ROS production more efficiently than saturated forms e.g., tocopherols [29].
In the present study, in DC reduced mean body weight at the end of experimental period but in DTT these were stable throughout experimental period. These findings are in agreement with related study [20] whose had demonstrated that, diabetic group without TRF supplementation showed significantly lower body weight than that of diabetic rat treated with TRF for 4 weeks.
While oral administration of d-δ-TRF for 3 weeks in DTT revealed reduced mean blood sugar level and in DC showed hyperglycemic state throughout the study period. These results correlate with other study [30] showing that the tocotrienol supplementation significantly increased the insulin levels and reduced the blood glucose in diabetic induced rats in dose dependent manner.
Macroscopic observation of healing wounds revealed remarkable changes in the wound size in treated groups even on14th day, suggestive of faster recovery in the treated groups. The reepithelialization in epidermis is widely accepted to be one of the major processes in wound healing that ensures successful repair [31–33]. Basal keratinocytes from both the wound edge and epidermal appendages such as hair follicles, sweat glands and sebaceous glands constitute the main sources for cells responsible for the reepithelialization [34].
Thickness of the epidermis is a good indicator for the superficial changes in the wound [22]. The mean values of histomorphological measurement in the present study showed that although the neoepidermis regenerated during the 2nd weeks in all groups, in treated groups the neoepidermal thickness was remarkably higher than the border epidermal thickness at the end of 2nd and 3rd weeks.
The global healing and remodeling indices (GHI and GRI) are used to measure the different stages of skin wound healing and its progress Lemo et al [22]. In cases of stronger wound remodeling the GRI can go up to 1. The mean values of GHI and GRI in the present study were high compared to control groups, indicating the positive therapeutic effects of d-δ-TRF in both healthy and diabetics.
Progression of wound healing revealed that while complete reepithelialization took 3 weeks in control group it took only two weeks in the treated group suggesting that d-δ-TRF promotes wound healing.
The interdigitations at dermoepidermal junction are known to provide both physical and trophic support. In treated groups well developed interdigitations appeared on entire length of neoepidermis on 2nd weeks and were well defined than those in the control groups on 3rd weeks. Therefore, the neoepidermis in treated groups has more capacity to resist the possibility of desquamations.
Dermal regeneration has been characterized by granulation tissue rich in fibroblasts, generally oriented parallel to the epidermal layer [25]. On 3rd weeks cellular components were more in control groups than treated groups. The fibroblasts were oval or spindle shaped and scattered in HC whereas in DC these cells were mainly stellate shaped, indicating incomplete dermal regeneration. In treated groups spindle shaped fibroblast lie parallel to the neoepidermis, suggesting that the d-δ-TRF supplementation helps the complete dermal regeneration.
Neovascularization is characterized by well-structured capillary vessels and absence of hemorrhage [25]. Numerous, well formed vertically oriented capillary vessels that run towards the epithelial surface were seen by the end of 3rd week in HC on whereas in treated groups these were observed by the end of 2nd week thus indicating an early and good neovascularization in the treated group. Swollen capillaries and extravasation of blood cells were seen in DC granulation tissue even on 3rd week, pointing towards its poor and delayed neovascularization. As remodeling progresses, there is a gradual reduction in the cellularity and vascularity of the reparative tissue [35]. This finding is supported by the present study as it indicated that the numbers of capillary vessels were reduced in treated groups on 3rd weeks.
The collagen fibres are mainly found in the papillary and reticular layers of the dermis and they provide both mechanical and structural integrity to the dermis [36]. In early phase of healing the collagen fibres in dermis revealed different orientation and packing density. On 2nd weeks collagen fibres were arranged as wavy pattern in DC. At the end of study period, in HC more fibres were obliquely placed while in DC the suprahypodermal area consists of poorly interlaced collagen fibres. In treated groups these fibres were horizontally placed and compactly interwoven and this horizontal alignment of collagen fibres indicates a better tissue remodeling [37].
Tough the elastin is a minor component of the dermis it has an important function in providing the elasticity of the skin [38]. At the end of experimental period, in control groups the elastin fibres appeared at the wound margins. In treated groups these structure were noticed one step advanced stage and newly formed smaller fibrils were diffusely arranged in the regenerating dermis. Presence of elastin fibres in the healing wound indicates final stages of matrix remodeling [39].
At the end of study period, in control groups hair follicles and sebaceous glands remained only at the wound margins. In treated groups hair follicles and sebaceous glands were in advance stage and had their made their presence into the regenerating dermis and even newly formed hairs were found within the hair follicles and on the neoepidermal surface, which indicated a faster healing and quicker remodeling of the wound matrix [37].
The predictors of cardiovascular complications in diabetes are believed to be dyslipidemia and hyperglycemia [40–43]. The present data indicated that mean values of total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL) and very low density lipoprotein (VLDL) levels were higher and high density lipoprotein (HDL) level lower in DC, indicating significant dyslipidemia in untreated diabetic rats [44]. The lower mean values of TC, TG, LDL and VLDL levels and high HDL level were recorded in DTT after 3 weeks treatments. This result is in agreement with related study [45].
The serum creatinine level is known to be a significant marker of diabetic nephropathy. Our result showed the serum creatinine level was higher in DC than all other groups and almost similar observation has been shown in the STZ-induced diabetic rat [44]. In DTT these level were improved after 3 weeks treatment and similar to the level of healthy groups (HC & HTT). The abnormally high level of serum creatinine was consistent with the impaired kidney function [46].
The total protein content is also known to be an indicator for the protein level and cellular proliferation of the wound tissue [47]. The result of present study also indicates that the d-δ-TRF treatment enhances protein synthesis in treated groups and its level lower in DC, which is in agreement with [44] who found that diabetic rats showed lower serum total protein level and when treated with vitamin E its level improves significantly.
Catalase is a preventive antioxidant which inhibits the initial production of free radicals. When H2O2 is generated in large quantities, the enzyme catalase is also used for its removal [48]. The present study showed that the serum catalase activity was lower in DC. Many other studies [49,50] stated that the catalase activity had decreased in plasma, liver and kidney of diabetic control rats. The decreased catalase activity in plasma and tissues of STZ-diabetic rats may be due to its increased utilization for scavenging the toxic products of lipid peroxidation or due to decreased availability of H2O2 [49]. Vitamin E treatment has been shown to normalize the catalase activity in the control group [50]. The result of present study revealed that d-δ-TRF supplementation enhances the serum catalase activity in treated groups.
Antioxidant capacity of plasma is the primary measure and marker to evaluate the status and potential of oxidative stress in the body [51]. Total antioxidant capacity has been shown to be significantly reduced in plasma and liver homogenate FRAP of diabetic rats compared to control animals [52–54]. The observation of present work with significantly lower (P<0.05) serum FRAP level in diabetic control compared to healthy control is in agreement with the findings of above mentioned workers. In treated groups, after 3 weeks supplementation with d-δ-TRF revealed the improved serum antioxidant capacity.
CONCLUSION
Based on findings of the present study it is concluded that d-δ-TRF promotes skin wound healing in both healthy and diabetic rats and thus indicative of its strong therapeutic potential in future in the management of skin wounds.
ACKNOWLEDGMENTS
All kinds of support availed from the Department of Anatomy, JN Medical College, Aligarh Muslim University is gratefully acknowledged.
Abbreviations
AF with FG: Aldehyde Fuchsin with Fast Green; DC: Diabetic Control; DTT: Diabetic d-δ-tocotrienol rich fraction treated; FRAP: Ferric Reducing Antioxidant Power; GHI: Global Healing Index; GRI: Global Remodeling Index; HC: Healthy Control; H&E: Haematoxylin & Eosin; HTT: Healthy d-δ-tocotrienol rich fraction treated; MT: Masson’s Trichrome; TAC: Total Antioxidant Capacity; PSR with FG: Picro Sirus Red with Fast Green.
REFERENCES
1. Latiff AA, Teoh S, Das S. Wound healing in diabetes mellitus: traditional treatment modalities. Clinica Terapeutica. 2010;161:359-64.
2. Karasu C, Ozansoy G, Bozkurt O, Erdo?an D, Omero?lu S. Antioxidant and Triglyceride-lowering effects of vitamin E associated with the prevention of abnormalities in the reactivity and morphology of aorta from streptozotocin-diabetic rats. Antioxidants in Diabetes-Induced Complications (ADIC) Study Group. Metabolism. 1997;46:872-79.
3. Goldman R. Growth factors and chronic wound healing: Past, present, and future. Adv. Skin Wound Care. 2004;17:24-35.
4. Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr. 1996;16:33-50.
5. Toshihiro K, Junichi F. Roles of Antioxidative Enzymes in Wound Healing. J Dev Biol. 2015;3:57-70.
6. Peerapatdit T, Likidlilid A, Patchanans N, Somkasetrin A. Antioxidant status and lipid peroxidation end products in patients of type 1 diabetes mellitus. J Med Assoc Thai. 2006;89:S141-46.
7. Suzuki YJ, Tsuchiya M, Wassall SR, Choo YM, Govil G, Kagan VE, Packer L. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: implication to the molecular mechanism of their antioxidant potency. Biochemistry. 1993;32:10692–9.
8. Ahsan H, Ahad A, Iqba J and Siddiqui WA. Pharmacological potential of tocotrienols: a review. Nutr Metab (Lond). 2014;52:1-22.
9. Packer L. Protective role of vitamin E in biological systems. Am J Clin Nutr. 1991;53:1050S-5S.
10. Elson CE. Tropical oils: Nutritional and scientific issues. Crit Rev Food Sci Nutr. 1992;31:79-102.
11. McIntyre BS, Briski KP, Tirmenstein MA, Fariss MW, Gapor A, Sylvester PW. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on normal mouse mammary epithelial cells. Lipids. 2000;35:171-80.
12. McIntyre BS, Briski KP, Gapor A, Sylvester PW. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on preneoplastic and neoplastic mouse mammary epithelial cells. Proc Soc Exp Biol Med. 2000;224:292-301.
13. Sylvester PW, McIntyre BS, Gapor A, Briski KP. Vitamin E inhibition of normal mammary epithelial cell growth is associated with a reduction in protein kinase C(alpha) activation. Cell Prolif. 2001;34:347-57.
14. Zaini AA, Khaza’ai H, Ali RM, Abdul Mutalib MS, Baharuddin AA. Topical Treatment of Tocotrienol-Rich Fraction (TRF) on Deep Partial-Thickness Burn Wounds in Rats. J Dermatolog Clin Res. 2016;4:1063-70.
15. Matough FA, Budin SB, Hamid ZA, Abdul-Rahman M, Al-Wahaibi N, Mohammed J. Tocotrienol-Rich Fraction from Palm Oil Prevents Oxidative Damage in Diabetic Rats. Sultan Qaboos University Med J. 2014;14:95-103.
16. Theriault A, Chao JT, Wang Q, Gapor A, Adeli K. Tocotrienol: a review of its therapeutic potential. Clin Biochem. 1999;32:309-19.
17. Musalmah M, Muhd Fairuz AH, Gapor MT and Wan Ngah WZ. Effect of tocotrienol-rich fraction on wound healing in streptozotocin-induced diabetic rats. Malaysian J Biochem Mol Biol. 2001;6:34-9.
18. Elsy B, Maheshwari V, Khan AA. Effects of d a-Tocopherol on Progression of Reepithelialization, Matrix Remodeling and Appearance of Epidermal Appendages in Secondary Skin Wounds of Diabetic Rats. J Dermatolog Clin Res. 2016;4:1081-7.
19. Sen CK, Khanna S, Rink C, Roy S. Tocotrienols: The emerging face of natural vitamin E. Vitam Horm. 2007;76:203-61.
20. Budin SB, Khairunnisa MY, Muhd Hanis MI, Zariyantey AH, Jamaludin M. Tocotrienol-rich fraction of palm oil reduced pancreatic damage and oxidative stress in streptozotocin-induced diabetic rats. Aust J Basic Appl Sci. 2011;5:2367-74.
21. Chen L, Mirza R, Kwon Y, DiPietro LA, Koh TJ. The murine excisional wound model: Contraction revisited. Wound Rep Reg. 2015;23:874-7.
22. Lemo N, Marignac G, Reyes-Gomez E, Lilin T, Crosaz O, Dohan Ehreenfest M. Cutaneous reepithelialization and wound contraction after skin biopsies in rabbit: a mathematical model for healing and remodelling matrix. Vet Arhiv. 2010;80:637-52.
23. Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389-94.
24. Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochem. 1996;239:70-6.
25. Altavilla D, Saitta A, Cucinotta D, Galeano M, Deodato B, Colonna M, et al. Inhibition of Lipid Peroxidation Restores Impaired Vascular Endothelial Growth Factor Expression and Stimulates Wound Healing and Angiogenesis in the Genetically Diabetic Mouse. Diabetes. 2001;50:667-74.
26. Senel O, Cetinkale O, Ozbay G, Ahc ioglu F, Bulan R. Oxygen free radicals impair wound healing in ischemic rat skin. Ann Plast Surg. 1997;39:517-23.
27. Rosenbaum MA, Miyazaki K and Graham LM. Hypercholesterolemia and oxidative stress inhibit endothelial cell healing after arterial injury. J Vasc Surg. 2012;55:489-96.
28. Hossam E, Osama MA, Ayman MM and Rasha RA. Limiting prolonged inflammation during proliferation and remodeling phases of wound healing in streptozotocin-induced diabetic rats supplemented with camel undenatured whey protein. BMC Immunol. 2013;14:31-44.
29. Sebastian S, Walter E M and Gunter PE. Tocotrienols: Constitutional Effects in Aging and Disease. Recent Advances in Nutritional Sciences. J Nutr. 2005;135:151-4.
30. Kuhad A, Bishnoi M, Tiwari V and Chopra K. Suppression of NF-kappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav. 2009;92:251-9.
31. Escamez MJ, Garcia M, Larcher F, Meana A, Munoz E, Jorcano JL et al. An in vivo model of wound healing in genetically modified skin-humanized mice. J Invest Dermatol. 2004;123:1182-91.
32. Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75-81.
33. Wysocki AB. Skin anatomy, physiology, and pathophysiology. Nurs Clin North Am. 1999;34:777-97.
34. DiPietro LA, Burns AL. Wound Healing: Methods and Protocols. Methods in Molecular Medicine. 2003; Totowa, N.J. Humana Press. Electronic book.
35. Peacock EE. Wound repair. In: Wound repair. Saunders, Philadelphia. 1984; 38-55.
36. Oikarinen A. Aging of the skin connective tissue: how to measure the biochemical and mechanical properties of aging dermis. Photodermatol Photoimmunol Photomed.1994;10:47-52.
37. Sushma RK, Pai SKR, Nayak JK, Hemalatha B, Keerthana P, Bhat MRK. Biomechanical, biochemical and histological evidences for wound healing properties of indian traditional medicines. Int J Pharm Pharm Sci. 2015;7:163-171.
38. Prost-Squarcioni C, Fraitag S, Heller M, Boehm N. Functional histology of dermis. Ann Dermatol Venereol. 2008;135:1S5-20.
39. Liora BW, Nessa S, Ram S, Tamar T. Novel Insights into Wound Healing Sequence of Events. Toxicol Pathol. 2007;35:767-79.
40. Chertow B, Edwards JC. Advances in Diabetes for the Milennium: Vitamins and Oxidant Stress in Diabetes and Its Complications. Medscape General Med. 2004;6:1-10.
41. Cullen P, Eckardstein A, Souris S, Schulte H. Assmann G. Dyslipidaemia and cardiovascular risk in diabetes. Diabetes Obes Metab. 1999;1:189-98.
42. Solano MPMD, Goldberg RBMD. Management of dyslipidemia in diabetes. Cardiol Rev. 2006;14:125-35.
43. Sout RW. Diabetes and athoresclerosis. Biomed Pharmacother. 2005;47:1-2.
44. Tavares de Almeida DA, pereira Braga C; Barbosa Novelli EL, Henrique Fernandes AA. Evaluation of Lipid Profile and Oxidative Stress in STZ Induced Rats Treated with Antioxidant Vitamin. Braz Arch Biol Technol. 2012;55:527-36.
45. Budin SB, Othman F, Louis SR, Bakar MA, Das S, Mohamed J. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics Sao Paulo. 2009;64:235-44.
46. Ronco C, Grammaticopoulos S, Rosner M, Decal M, Soni, S, Lentini P. Oliguria. Creatinine and other biomarkers of acute kidney injury. Contributions Nephrol. 2010;64:118-27.
47. Teoh SL, Latiff AA, Das S. The effect of topical extract of Momordica charantia (bitter gourd) on wound healing in nondiabetic rats and in rats with diabetes induced by streptozotocin. Clin Exp Dermatol. 2009;34:815-22.
48. Vasudevan DM and Sreekumari S. Textbook of Biochemistry (For Medical Students), 4th edition. 2005; 340-1
49. Jeyashanthi N, Ashok V. Anti-Oxidative Effect of Cassia auriculata on Streptozotocin Induced Diabetic Rats. Ind J Clin Biochem. 2010;25:429-34.
50. Shirpoor A, Khadem Ansari MH, Salami S, Ghaderi Pakdel F, Rasmi Y. Effect of vitamin E on oxidative stress status in small intestine of diabetic rat. World J Gastroenterol. 2007;13:4340-4.
51. Brahm KT, Kanti BP, Abidi AB, Syed Ibrahim R. Markers of Oxidative Stress during Diabetes Mellitus. J Biomark. 2013;2013:378790.
52. Cakatay U, Kayali R. The evaluation of altered redox status in plasma and mitochondria of acute and chronic diabetic rats. Clin Biochem. 2006;39:907-12.
53. Soliman GZA, Bahagt NM. Effect of vitamin C and/or vitamin E on oxidative stress and lipid profi le in diabetic rats. Res J Pharm Biol Chem Scien. 2012;3:639-52.
54. Alireza N, Bokaeian M, Mohsen S, Ali F, Azim A. Attenuation of oxidative stress in streptozotocin-induced diabetic rats by eucalyptus globulus. Indian J Clin Biochem. 2009;24:419-25.
Notes
Source of Support: Nil
Conflict of Interest: None declared.

Comments are closed.