|
Get Citation
|
|
|
Elsy B, Khan AA, Maheshwari V. Effect of vitamin E isoforms on the primary intention skin wound healing of diabetic rats. Our Dermatol Online. 2017;8(4):369-375. |
|
|
Download citation file:
|
Effect of vitamin E isoforms on the primary intention skin wound healing of diabetic rats
Bijo Elsy1, Aijaz Ahmed Khan1, Veena Maheshwari2
1Department of Anatomy, JN Medical College, Aligarh Muslim University, Aligarh, India; 2Department of Pathology, JN Medical College, Aligarh Muslim University, Aligarh, India
Corresponding author: Dr. Bijo Elsy, E-mail: bijobaby22@yahoo.com
Submission: 04.04.2017; Acceptance: 20.06.2017
DOI: 10.7241/ourd.20174.108
ABSTRACT
Introduction: Impaired wound healing events is a common complication in diabetes. One of the effective nutritional antioxidant on skin wound healing is vitamin E which contains saturated tocopherol and unsaturated tocotrienol forms. This present study is designed to explore the effect of different vitamin E isoforms on stitched skin wound in both healthy and diabetic rats.
Materials and Methods: Forty eight albino rats were divided into eight groups; healthy control, diabetic control, healthy treated (d-α-tocopherol, d-δ-TRF and co-administrated) and diabetic treated (d-α-tocopherol, d-δ-TRF and co-administrated). Diabetes was induced through single subcutaneous injection of alloxan at the dose of 100 mg/kg. Treated groups were administered d-a-tocopherol (200 mg/kg), d-δ-TRF (200 mg/kg) and co-administration (100 mg/kg of these two compounds each) orally and daily for three weeks. A horizontal skin incision was made on right mid-thigh region at 2.95 ± 0.17cm in length and wound was closed with an absorbable suture.
Results: Histopathological and histomorphological results at the end of 3rd week revealed that the d-δ-TRF treated groups promote the regeneration and reorganization of epidermal and dermal components in healing of primary intention more effectively than the d-α-tocopherol and co-administrated groups.
Conclusion: It is concluded that among different vitamin E isoforms the d-δ-TRF appears to be a more effective nutritional antioxidant on skin wound healing in both healthy and diabetics.
Key words: Antioxidant; d-a-tocopherol; d-δ-TRF; Diabetes; Rats; Skin; Wound
INTRODUCTION
In diabetes the free radicals impair the normal wound healing by damaging keratinocyte, endothelial cells, capillary permeability and collagen metabolism [1]. Oxidative stress induces cellular dysfunction and retards angiogenesis and the healing process [2]. Thus, elimination of reactive oxygen species (ROS) is an important strategy to improve the healing of wounds in diabetes mellitus patients [3].
The unsaturated tocotrienol forms of vitamin E are more potent antioxidants [4] and suppress ROS production more efficiently than most active saturated tocopherol forms [5,6]. Each isoforms of vitamin E have different biological activities towards free radicals [7]. In addition tocotrienol possess antidiabetic and anticancer properties as well [8]. Interestingly, the antitumor activity of tocotrienols is not dependent on its antioxidant activity [9,10]. The highly biopotent γ and δ- tocotrienols may play a physiological role in modulating normal cell growth, function and remodeling. These compounds inhibit tumor growth without harming normal tissues [11–14].
Sutures enhance wound closure and promote healing. Sutures initially provide the mechanical strength to seal the wound and protect it from pathogens [15]. Available studies [16,17] revealed that topically applied vitamin E does not help in improving the cosmetic appearance of scars or its failure to reduce postoperative scar formation. In a 10 days study [18] it has shown that topical tocopherol treatment enhances the rate of secondary skin wound closure in streptozotocin-induced diabetic rat.
According to Zaini et al [19], the tocotrienol-rich fraction (TRF) treatment accelerate the wound contraction rate, enhance the reepithelialization, the regeneration process and stimulate the granulation tissue formation in deep partial-thickness burn wounds. In another study [20] it was revealed that the supplementation of TRF at 200 mg/kg was able to improve wound healing in type 1 induced diabetic rat.
Our previous studies [21–23] explain the effect of different vitamin E isoforms via single and co-administrations on secondary skin wound healing and from these studies it was concluded that the d-δ-TRF treated group showed comparatively faster recovery and regeneration of epidermal and dermal components than other treated and control groups.
At present all available data on vitamin E application on skin wound healing either topical application on primary intention of healing or oral administration on secondary intention of healing. To our knowledge, so far no information is available on oral administration of vitamin E isoforms on primary intention type of skin wound healing. Hence the present study was attempted to explore the effect of oral administration of different isoforms of vitamin E on the basis of histopathological characteristics and histomorphological measurements of incised skin wounds in both healthy and diabetic rats closed with absorbable suture in experimental surgery.
MATERIALS AND METHODS
Forty eight albino rats of either sex each weighing 230-320g was obtained from central animal house of JN medical college, AMU, Aligarh. The study has been approved by Institutional Animal Ethical Committee (No. 8937/2014).
This present study followed the same method as described in our previous studies [21,22] of animal care, induction of diabetes, monitoring of blood sugar level, surgical procedure, tissue sample collections and fixation.
Experimental Groups, Route and Dosage of Treatment
Total forty eight animals were divided into eight groups having six rats in each group: (1) healthy control- HC; (2) diabetic control- DC; (3) healthy d-α-tocopherol treated- HPT and (4) diabetic d-α-tocopherol treated- DPT; (5) healthy d-δ-TRF treated- HTT and (6) diabetic d-δ-TRF treated – DTT; (7) healthy d-a-tocopherol and d-δ-TRF treated- HXT and (8) diabetic d-α-tocopherol and d-δ-TRF treated- DXT.
The d-α-tocopherol treated groups were received 200mg/kg d-α-Tocopherol (Myra e capsule [Vitamin E] manufactured by PT Daya- Baria laboratoria Tbk, Indonesia; Imported and packed by United laboratories, Inc, 66 United St, Philippines). The d-δ-TRF treated groups were received 200mg/kg d-δ-TRF (Unique E Tocotrienol, tocopherol free, 90% δ and 10% γ tocotrienols, AC Grace Company, P.O Box 570, Big Sandy, TX 75755, USA). The co-administrated groups were received 100mg/kg of d-α-tocopherol and d-δ-TRF each.
All treated groups were supplemented vitamin E isoforms (d-a-tocopherol and d-δ-TRF) daily for three weeks by oral administration.
Surgical Procedure
All animals received general anesthesia via inhalation of ether. Horizontal skin incision was made on the shaved right mid-thigh region at 2.95 ± 0.17cm in length. Skin were closed with 3-0 Vicryl (2metric–NW2401) absorbable sterilized surgical needled suture USP (synthetic; braided coated polyglactin 910 violet; from Ethicon, manufactured in India by Johnson and Johnson Ltd, Aurangabad). Povidone-iodine solution (antisepsis) was applied on the wound and 0.5 ml Voveran (analgesic) and 2 mg single shot of Gentamycin (antibiotic) were also injected simultaneously.
Sample Collection and Fixation of Tissue
On completion of three weeks animals were sacrificed under deep ether anesthesia and then excised the healed parts of skin with adjacent area. The excised tissues were immersion-fixed in 10% neutral buffered formalin
Macroscopic Examination
The macroscopic changes in the wound healing stages were observed and recorded photographically on 1st, 7th, 14th & 21st day of creation of wounds.
Histopathology and Histomorphometry
Fixed tissue samples were processed for light microscopical studies. The 5m thick paraffin sections were stained with Haematoxylin & Eosin (H&E), Masson’s Trichrome (MT) and Aldehyde Fuchsin with Fast Green (AF with FG).
In histomorphometry the measurements of epidermal and neoepidermal thickness were performed on the H & E and MT stained sections by using software Motic images plus version 2.0.
Statistical Analysis
Histomorphological measurements were statistically evaluated and the significance calculated by using one way ‘ANOVA’ followed by Tukeys test. All the results were expressed as mean ± SD and P < 0.05 was considered as statistically significant.
RESULTS
Body Weight and Blood Sugar Level
Weight and blood sugar levels of all animals in each group were monitored at weekly intervals and results were reported in previous studies [21–23].
Macroscopic Observations
On 3rd week scar were not formed in any groups by morphological examination on stitched skin wounds and better wound healing was observed in all treated groups especially in d-δ-TRF treated group showed almost complete healing as compared to control groups (Fig. 1).
Microscopic Observations
Histomorphometry
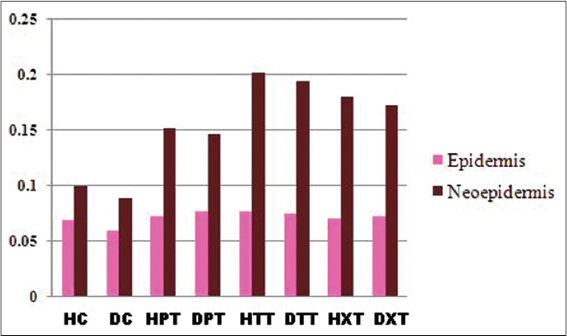
In treated groups the mean values of neoepidermis were significantly thicker (P<0.01) than the corresponding epidermal border thickness which in the order of d-δ-TRF> co-administrated> d-α-tocopherol groups as compared to control groups (Fig. 2).
Reepithelialization
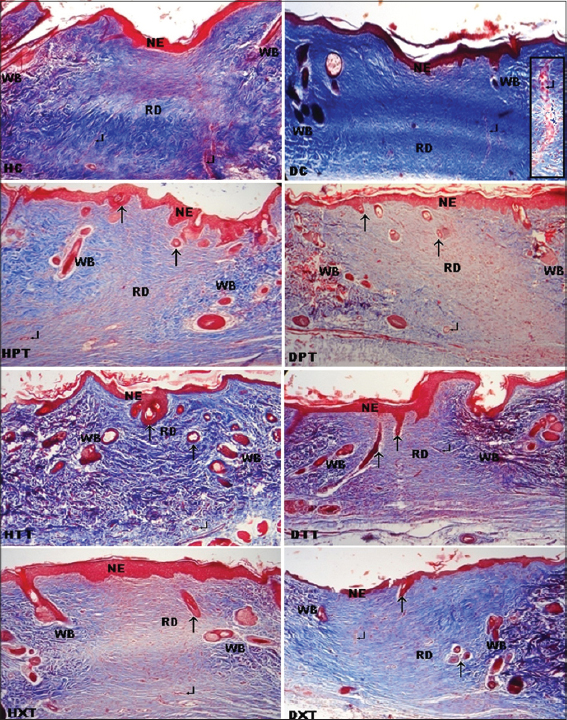
Complete reepithelialization was noticed in all groups (controls and treated). On 3rd weeks, all groups were showed interdigitations but well defined interdigitations at dermoepidermal junction appeared on the entire length of neoepidermis were seen only in treated groups (Fig. 3).
Cellular components
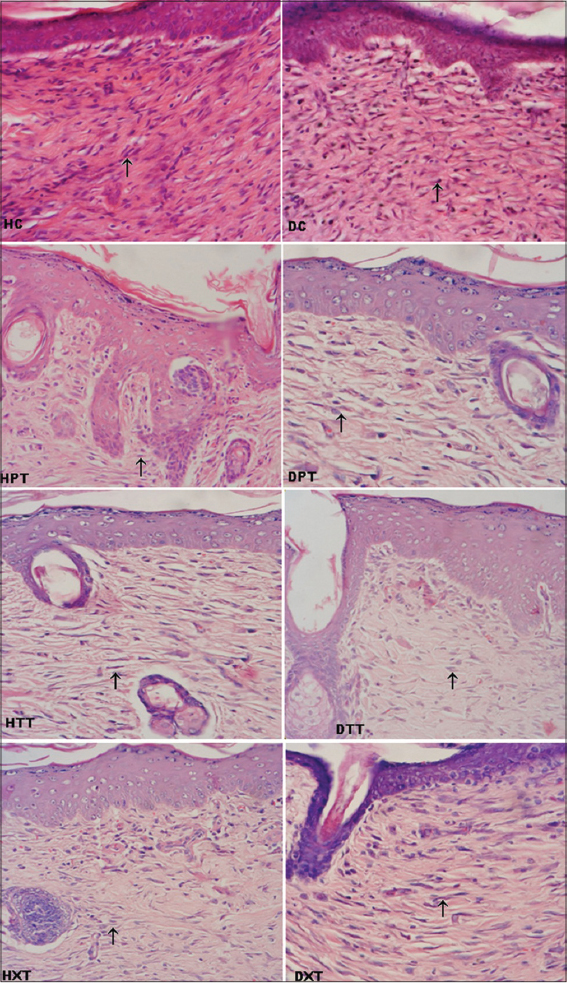
On 3rd week more cellularity were observed in control groups as compared to all treated groups. Among different treated groups reduced cellularity were seen in d-δ-TRF treated group than d-a-tocopherol and co-administrated treated groups (Fig. 4).
Neovascularization
Reduced vascularity was observed in all treated groups. In HC has shown more vertically oriented blood capillaries whereas in DC blood capillaries were swollen (Fig. 3).
Matrix remodeling and skin appendages
On 3rd weeks, in all treated groups the collagen fibres in the regenerated dermis were mostly horizontally arranged and compactly interwoven especially in HTT most of the collagen fibres were matured but these fibres were more thickened which form fibrosis in control groups (Fig. 3).
Well defined elastin fibres were seen in the area of regenerating dermis and nearby this area in all treated groups and reappearance of these fibres in the order of d-δ-TRF> co-administrated> d-α-tocopherol groups. Whereas in control groups these fibres were seen only at wound margins (Fig. 5).
At the end of study period, more hair follicles with hairs and sebaceous glands were observed in regenerating dermis and neoepidermis of all treated groups which in the order of d-δ-TRF>d-α-tocopherol> co-administrated groups. But in control groups these features were restricted at the wound margins (Figs 3 and 4).
DISCUSSION
Impeded wound healing is now a well-known phenomenon in both experimental and clinical diabetes [24]. Hyperglycemia is known to cause increased production of free radicals and insufficiencies in the antioxidant system [25]. The antioxidants have the ability to reduce the diabetic complications by arresting free radical-induced damage [26]. Vitamin E is a family of essential micronutrients [27] and its one of the most popular applications are in the treatment of burns, surgical scars and wounds [28].
Macroscopic examinations healing wounds on 3rd week revealed that scar was not formed in any groups. However, a better and faster wound healing was observed in d-δ-TRF treated group followed by d-α-tocopherol treated and co-administrated groups as compared to control groups.
Good marker for superficial changes in the wound is the epidermal thickness [29]. The mean values of histomorphological measurement in the present study showed that on 3rd week in d-δ-TRF group the neoepidermal thickness was remarkably higher than the corresponding border epidermal thickness compared to all other groups. But in co-administrated groups it was thicker than the respective border epidermis of d-α-tocopherol and control groups.
The histopathological observations have shown complete reepithelialization and interdigitations in all groups. But well defined interdigitations on the entire length of neoepidermis appeared only in treated groups. The interdigitations at dermoepidermal junction are known to provide both physical and trophic support. Therefore, the neoepidermis in treated groups has obviously more capacity to resist the possibility of desquamations [21–23].
Presence of more fibroblasts in the granulation tissues is an indicator of dermal regeneration [24] but reduced cellularity indicates that the dermal components are in advanced stages of remodeling [30]. On 3rd weeks cellular components were more in control groups whereas these features were reduced in d-δ-TRF group than d-a-tocopherol and co-administrated groups. These results suggest that the dermal regeneration process was slow in control groups but the three weeks d-δ-TRF supplementation boost the early dermal regeneration as compared to d-α-tocopherol supplementation and co-administration.
Well-structured capillary vessels with absence of hemorrhage are the characteristic feature of neovascularization [24]. More vertically oriented capillary vessels that run towards the epithelial surface were seen in HC whereas in DC blood capillaries were swollen. Reduced vascularity in the reparative tissue is an indictor of dermal remodeling [30]. This finding is supported by the present study as it indicated that the numbers of blood capillary vessels were reduced in all treated groups. These results were also in agreement with our previous studies [21–23].
The collagen fibres are mainly found in the papillary and reticular layers of the dermis and they provide both mechanical and structural integrity to the dermis [31]. At the end of study period, in both control groups the collagen fibres were thicker in the scar tissue. In all treated groups collagen fibres were horizontally placed and compactly interwoven and in addition to these features in HTT most of the collagen fibres were mature, a feature similar to our previous studies [21–23] on secondary skin wound healing. The horizontal alignment of collagen fibres suggests a better tissue remodeling [32].
Tough the elastin is a minor component of the dermis it has an important function in providing the elasticity of the skin [33]. Well defined elastin fibres were seen in the regenerating dermis and its nearby area in all treated groups, plenty of these fibres reappeared in d-δ-TRF treated group followed by co-administrated group compared to d-α-tocopherol treated group. But in control groups these fibres were observed only at wound margins. Same observations were found in our previous studies [21–23] of secondary skin wound healing. Presence of elastin fibres in the healing wound indicates final stages of matrix remodeling [34].
Presence of epidermal appendages such as hair follicles and sebaceous glands in the regenerating dermis and neoepidermis indicate the faster healing and quicker remodeling of wound matrix [32]. At the end of study period, more hair follicles with hairs and sebaceous glands were observed in all treated groups whereas these features were restricted at wound margins in control groups. This study is in agreement with our previous studies [21–23] that among different isoforms of vitamin E, the d-δ-TRF has potency to accelerate the matrix remodeling more effectively than d-α-tocopherol and co-administration of both these isoforms. In matrix remodeling the d-α-tocopherol treated group had shown better results than co-administrated groups.
CONCLUSION
Based on histopathological and histomorphological results it is concluded that the d-δ-TRF accelerates the regeneration and reorganization of epidermal and dermal components in healing of primary intention more effectively than the d-α-tocopherol and co-administration. Therefore among these different vitamin E isoforms the oral administration of d-δ-TRF is a most potent nutritional adjuvant on incisional skin wound healing in both healthy and diabetics.
ACKNOWLEDGMENTS
All kinds of support availed from the Department of Anatomy, JN Medical College, Aligarh Muslim University is gratefully acknowledged.
REFERENCES
1. Senel O, Cetinkale O, Ozbay G, Ahcioglu F, Bulan R. Oxygen free radicals impair wound healing in ischemic rat skin. Ann Plast Surg. 1997;39:517-23.
2. Rosenbaum MA, Miyazaki K, Graham LM. Hypercholesterolemia and oxidative stress inhibit endothelial cell healing after arterial injury. J Vasc Surg. 2012;55:489-96.
3. Hossam E, Osama MA, Ayman MM, Rasha RA. Limiting prolonged inflammation during proliferation and remodeling phases of wound healing in streptozotocin-induced diabetic rats supplemented with camel undenatured whey protein. BMC Immunol. 2013;14:31-44.
4. Serbinova EA, Kagan VE, Han D, Packer L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic Biol Med. 1991;10:263-75.
5. Sebastian S, Walter EM, Gunter PE. Tocotrienols: Constitutional Effects in Aging and Disease. Recent Advances in Nutritional Sciences. J Nutr. 2005;135:151-4.
6. Thiele JJ, Hsieh SN, Ekanayake-Mudiyanselage S. Vitamin E: critical review of its current use in cosmetic and clinical dermatology. Dermatol Surg. 2005;31:805-13.
7. Yoshida Y, Niki E, Noguchi N. Comparative study on the action of tocopherols and tocotrienols as antioxidants: chemical and physical effects. Chem Phys Lipids 2003;123:63-75.
8. Ahsan H, Ahad A, Iqba J, Siddiqui WA. Pharmacological potential of tocotrienols: a review. Nutr Metab (Lond). 2014;52:1-22.
9. Packer L. Protective role of vitamin E in biological systems. Am J Clin Nutr. 1991;53:1050S-5S.
10. Elson CE. Tropical oils: Nutritional and scientific issues. Crit Rev Food Sci Nutr. 1992;31:79-102.
11. McIntyre BS, Briski KP, Tirmenstein MA, Fariss MW, Gapor A, Sylvester PW. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on normal mouse mammary epithelial cells. Lipids. 2000;35:171-80.
12. McIntyre BS, Briski KP, Gapor A, Sylvester PW. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on preneoplastic and neoplastic mouse mammary epithelial cells. Proc Soc Exp Biol Med. 2000; 224:292-301.
13. Sylvester PW, McIntyre BS, Gapor A, Briski KP. Vitamin E inhibition of normal mammary epithelial cell growth is associated with a reduction in protein kinase C(alpha) activation. Cell Prolif. 2001;34:347-57.
14. Hiura Y, Tachibana H, Arakawa R, Aoyama N, Okabe M, Sakai M, et al. Specific accumulation of γ- and δ-tocotrienols in tumor and their antitumor effect in vivo. J Nutr Biochem. 2009;20:607-13.
15. Yang CS, Chen CY, Chiang CH, Tung CL, Chen MY, Yeh CH, et al. The Effect of Suture Size on Skin Wound Healing Strength in Rats. J Med Biol Eng. 2010;31:339-43.
16. Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg.1999;25:311-5.
17. Jenkins M, Alexander JW, MacMillan BG, Waymack JP, Kopcha R. Failure of topical steroids and vitamin E to reduce postoperative scar formation following reconstructive surgery. J Burn Care Rehabil. 1986;7:309-12.
18. Teoh SL, Latiff AA, Abd Hamid NA, Wan Ngah WZ bt, Musalmah M. Evaluation of Topical Tocopherol Cream on Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Rats. Evid Based Complement Alternat Med. 2012;491027.
19. Zaini AA, Khaza’ai H, Ali RM, Abdul Mutalib MS, Baharuddin AA. Topical Treatment of Tocotrienol-Rich Fraction (TRF) on Deep Partial-Thickness Burn Wounds in Rats. J Dermatolog Clin Res. 2016 4:1063-70.
20. Musalmah M, Muhd Fairuz AH, Gapor MT, Wan Ngah WZ. Effect of tocotrienol-rich fraction on wound healing in streptozotocin-induced diabetic rats. Malaysian J Biochem Mol Biol. 2001;6:34-9.
21. Elsy B, Maheshwari V, Khan AA. Effects of d-α-Tocopherol on Progression of Reepithelialization, Matrix Remodeling and Appearance of Epidermal Appendages in Secondary Skin Wounds of Diabetic Rats. J Dermatolog Clin Res. 2016;4:1081-7.
22. Elsy B, Khan AA, Maheshwari V. Therapeutic potential of d-δ-tocotrienol rich fraction on excisional skin wounds in diabetic rats. Our Dermatol Online. 2017;8:1-9 (In press).
23. Elsy B, Khan AA, Maheshwari V. Effect of co-administration of vitamin E isoforms d-a-tocopherol and d-δ-tocotrienol rich fraction on the healing of skin wounds in diabetic rats. Int J Clin Dermatol. 2017;1:5-15.
24. Altavilla D, Saitta A, Cucinotta D, Galeano M, Deodato B, Colonna M, et al. Inhibition of Lipid Peroxidation Restores Impaired Vascular Endothelial Growth Factor Expression and Stimulates Wound Healing and Angiogenesis in the Genetically Diabetic Mouse. Diabetes. 2001;50:667-74.
25. Karasu C, Ozansoy G, Bozkurt O, Erdo?an D, Omero?lu S. Antioxidant and Triglyceride-lowering effects of vitamin E associated with the prevention of abnormalities in the reactivity and morphology of aorta from streptozotocin-diabetic rats. Antioxidants in Diabetes-Induced Complications (ADIC) Study Group. Metabolism. 1997;46:872-79.
26. Peerapatdit T, Likidlilid A, Patchanans N, Somkasetrin A. Antioxidant status and lipid peroxidation end products in patients of type 1 diabetes mellitus. J Med Assoc Thai. 2006;89:S141-46.
27. Pereira GG, Guterres SS, Balducci AG, Colombo P, Sonvico F. Polymeric Films Loaded with Vitamin E and Aloe vera for Topical Application in the Treatment of Burn Wounds. Biomed Res Int. 2014;641590:1-9.
28. Keen MA, Hassan I. Vitamin E in dermatology. Ind Dermatol Online J. 2016;7:311-5.
29. Lemo N, Marignac G, Reyes-Gomez E, Lilin T, Crosaz O, Dohan Ehreenfest M. Cutaneous reepithelialization and wound contraction after skin biopsies in rabbit: a mathematical model for healing and remodelling matrix. Vet Arhiv. 2010;80:637-52.
30. Peacock EE. Wound repair. In: Wound repair. Saunders, Philadelphia. 1984;38-55.
31. Oikarinen A. Aging of the skin connective tissue: how to measure the biochemical and mechanical properties of aging dermis. Photodermatol Photoimmunol Photomed. 1994;10:47-52.
32. Sushma RK, Pai SKR, Nayak JK, Hemalatha B, Keerthana P, Bhat MRK. Biomechanical, biochemical and histological evidences for wound healing properties of Indian traditional medicines. Int J Pharm Pharm Sci. 2015;7:163-71.
33. Prost-Squarcioni C, Fraitag S, Heller M, Boehm N. Functional histology of dermis. Ann Dermatol Venereol. 2008;135:1S5-20.
34. Liora BW, Nessa S, Ram S, Tamar T. Novel Insights into Wound Healing Sequence of Events. Toxicol Pathol. 2007;35:767-79.
Notes
Source of Support: Nil
Conflict of Interest: None declared.


Comments are closed.