|
Get Citation
|
|
|
Bartoš V, Kullová M. Cutaneous squamous cell carcinoma in situ with extensive clear cell change – A report of two cases. Our Dermatol Online. 2017;8(3):311-314. |
|
|
Download citation file:
|
Cutaneous squamous cell carcinoma in situ with extensive clear cell change – A report of two cases
Vladimír Bartoš1, Milada Kullová2
1Department of Pathology, Faculty Hospital in Žilina, V. Spanyola 43, Žilina, 012 07, Slovakia; 2Department of Dermatovenerology, Faculty Hospital in Žilina, V. Spanyola 43, Žilina, 012 07, Slovakia
Corresponding author: Dr. Vladimír Bartoš, MD PhD, E-mail: vladim.bartos@gmail.com
Submission: 20.09.2016; Acceptance: 21.11.2016
DOI: 10.7241/ourd.20173.89
ABSTRACT
Squamous cell carcinoma (SCC) of the skin includes many histologic subtypes with varying microscopic features and clinical behaviors. Clear cell SCC belongs to one of the rare histologic variants of this cutaneous malignancy. Herein, two cases are described in a 78-year- old man arising on the cheek and in a 79-year-old woman arising on the neck. In both examples, histology revealed an intraepidermal (in situ) SCC with a predominant clear cell change of malignant keratinocytes. In addition, a typical keratinizing squamous cell component was also present. Clear cell population showed apparent mitotic and proliferative activity. In one patient, it slightly stained with periodic acid-Shiff (PAS) method. Staining with alcian blue was negative in both cases. Knowledge on histomorphology and pathogenesis of this tumor entity is discussed.
Key words: Squamous cell carcinoma, Histological subtypes, Clear cell change
INTRODUCTION
Squamous cell carcinoma (SCC) of the skin includes many histologic subtypes and variants with varying microscopic features and clinical behaviors. Despite this broad diversity, the overall tendency for pathologists and clinicians alike is to refer to all epithelial neoplasms with predominantly squamous differentiation simply as SCC, with no further specification [1]. Clear cell SCC (whether intraepidermal or invasive) belongs to one of the rare histologic variants of this common cutaneous malignancy [1,2]. Like most other SCCs, it predominantly occurs on permanently sun-exposed areas, especially on the head and neck in elderly people [2]. Histologically, it is primarily composed of neoplastic keratinocytes with abundant pale cytoplasm. Apart from this component with clear cell appearance, the tumors may show foci of conventional SCC that point to the real nature of the neoplasm [1,2]. Nowadays, etiopathogenetic mechanism of clear cells formation in cutaneous SCC is not fully elucidated. Herein, we briefly report two cases of primary SCC of the skin with extensive clear cell change.
CASE REPORTS
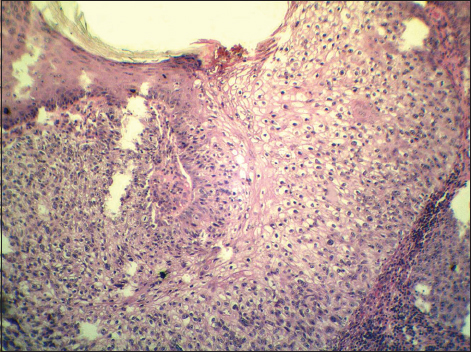
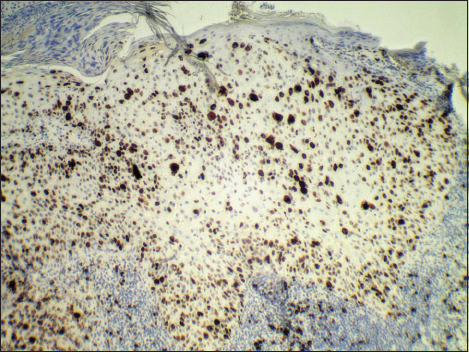
The first patient was a 78-year-old man who manifested with a slightly protuberated skin tumor on the left cheek over the mandible. The lesion was gray-brownish, poorly demarcated and measured approximately 35×25 mm. It was focally eroded with a crusto-squamous debris adhered on the surface. In the affected skin area, the rosacea changes were also grossly visible. A presumptive clinical diagnosis of basal cell carcinoma was made. A probatory biopsy of tumor was done and the sample was send for histological investigation. Hematoxylin and eosin (H&E) stained paraffin sections confirmed an intraepidermal (in situ) SCC with a predominant clear cell change of malignant keratinocytes (Fig. 1). These clear cells had a polyhedral shape and comprised about 90% of the entire tumor mass. In addition, typical squamous cell component with reticulated eosinophilic cytoplasm and abortive keratinisation was also seen (Fig. 2). Both, squamous and clear cell components were mixed without distinct boundaries. Immunohistochemical studies showed positive reaction for high molecular weight cytokeratins (clone 34bE12, DAKO), patchy expression of epithelial membrane antigen (clone E29, DAKO) and negative staining for epithelial antigen (clone BerEP4, DAKO). Clear cell population slightly stained with with periodic acid-Shiff (PAS) method (Fig. 3), but not with alcian blue. In the most actively proliferative areas, more than 10 mitotic figures per 10 high power files were counted, and Ki-67 antigen (clone MM1, LEICA) exceeded 50% (Fig. 4). Surrounding skin tissue exhibited a solar damage and intense chronic inflamation, but without actinic keratosis. The corium was free of neoplastic structures. After final diagnosis, the lesion was treated by local radiotherapy (18×3 Gy). No tumor relapse has been recognized.
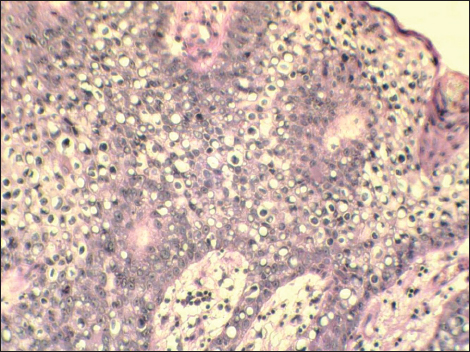
The second patient was a 79-year-old woman who presented with an elevated skin tumor resistence in the right side of the neck below the auricle. The lesion was pink-reddish, rounded, well-defined with 8 mm in diameter. On the surface, thick scales and crust were present. The clinical impression was basal cell carcinoma. A total surgical extirpation was carried out and a specimen was send for microscopic examination. Histologic sections revealed an intraepidermal (in situ) SCC composed primarily of rounded neoplastic cells with a clear cytoplasm, somewhere mimicking a signet-ring appearance (Fig. 5). The amount of these cells constituted about 80% of the tumor mass. They were diffusely mixed with malignant keratinocytes with keratinizing squamous and basaloid squamous appearance. Immunohistochemically, the tumor was also positive for high molecular weight cytokeratins (clone 34bE12, DAKO) and epithelial membrane antigen (clone E29, DAKO). However, clear cell population did not stained with periodic acid-Shiff (PAS) method or alcian blue. The mitotic rate reached 20 mitoses per 10 high power fields in areas of maximum proliferation. Surrounding skin tissue showed a mild solar damage and chronic inflamation, but without actinic keratosis. The corium was free of tumor. Resection margins were intact and after surgery, no local recurrence has been observed.
DISCUSSION
Clear cell SCC of the skin was first described by Kuo in 1980 [3] with a collection of six cases comprised of three histologic types: keratinizing (type I), nonkeratinizing (type II), and pleomorphic (type III). Type I was characterized by sheets or islands of tumor cells with empty-appearing cytoplasm, accompanied by foci of keratinization or keratin pearl formation. Type II demonstrated parallel or anastomosing cords of neoplastic clear cells in a compressed fibrotic stroma, but without evidence of keratinization. Type III tumors were described as pleomorphic neoplasms with clear cells arising from the epidermis, which show foci of squamous differentiation, dyskeratotic cells, and acantholysis and usually perineural and vascular involvement.
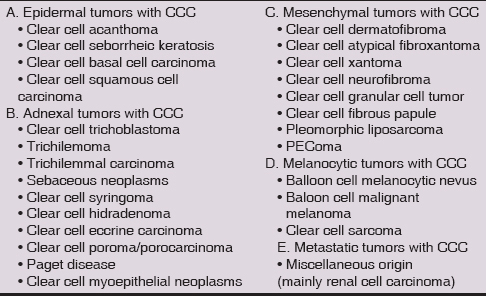
Current data on incidence and histogenesis of clear cell cutaneous SCC are insufficient. Generally, this variant is sporadically diagnosed and described only in a few reports, including clear cell Bowen’s disease [4–8]. Moreover, since conventional SCCs occasionally also exhibit clear cell change, there is no consensus, from what proportion of the clear cells may a lesion be classified as clear cell SCC. Some authors [9] suggested that clear cells were generally common in SCCs, though only some lesions presented a large number of them. Among 229 cutaneous SCCs, Kuo [3] originally diagnosed merely 6 clear cell variants (2.6%), but all of them consisted almost entirely of clear cell population. All other cases, in which those cells occurred just focally, were not considered clear cell variant of SCC. In similar study, Corbalán-Vélez et al. [9] analysed a set of 249 cutaneous SCCs and found tumor cells showing a clear cytoplasm in over half of the cases. However, only 7 lesions (3%) contained more than 75% of clear cell population. This percentage of tumors with a large amount of clear cells was similar to that found by Kuo [3]. Another authors [10] considered in situ clear cell SCCs only those ones, in which clear cell population exceeded 80% of tumor mass. Regardless of the terminology, due to potential diagnostic difficulties, the pathologists should be aware of this unusual microscopic feature in SCC. Since a spectrum of cutaneous neoplasms, that may histologically show striking clear cell change is very wide (Table 1), for making an accurate diagnosis, a complex differential-diagnostic approach is necessary, especially in a limited biopsy specimen.
An etiology of clear cell change in cutaneous SCC is still matter of debate. Original Kuo’s examples showed no evidence of either glycogen or mucin within the tumor cells which would support his hypothesis that clear cell change is a degenerative phenomenon caused by accumulation of intracellular fluid. However, in a more recent retrospective study of 40 cases of SCC with clear cells [12], only two lacked glycogen accumulation in their cytoplasm. It has been speculated in certain papers [9,10] that clear cell change in cutaneous SCC may be associated with adnexal (outer root sheath) differentiation. It has been also suggested [11], that some of the published cases of clear cell SCC may arguably be classified as trichilemmal carcinoma and vice versa. Even Misago & Ackerman [13] believed that what conventionally is called trichilemmal carcinoma is a clear cell variant of SCC. In our examples, only one lesion showed intracytoplasmic PAS-positivity suggesting an accumulation of glycogen, whereas the second one was PAS-negative. Of interest, neoplastic clear cell population did not seem to have the same appearance in both lesions. While in the first patient, these cells have a polyhedral shape and grew compactly, in the second person, they were rounded and dispersely mixed with malignant keratinocytes of non-clear appearance. Anyway, none of the lesions displayed the features of trichilemmal differentiation (peripheral palisading of columnar clear cells resting on a hyaline basament membrane, with their nuclei located at the opposite pole).
In conclusion, cutaneous SCC with striking clear cell change is a rare entity found in a routine dermatopathological practice. Despite of this some authors argue [9] it may be underdiagnosed and be more frequent than the literature suggests. It is possible that etiopathogenesis of clear cells formation in SCC may be diverse and various mechanisms, e.g. hydropic degeneration, accumulation of polysaccharides or koilocytic transformation, result in this unusual microscopic feature. In such case, clear cell SCC may in fact be a denominator comprising a heterogeneous group of lesions with a different biologic behaviour and malignant potential.
Consent
The examination of the patients was conducted according to the Declaration of Helsinki principles.
REFERENCES
1. Cassarino DS, Derienzo DP, Barr JR. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. Part two. J Cutan Pathol. 2006;33:261-79.
2. Rongioletti F, Margaritescu I, Smoller BR. Rare malignant skin tumors. Springer New York, Heilderberg, Dordrecht, London. 2015.
3. Kuo T. Clear cell carcinoma of the skin: a variant of the squamous cell carcinoma that stimulates sebaceous carcinoma. Am J Surg Pathol. 1980;4:573583.
4. Lawal AO, Adisa AO, Olajide MA, Olusanya AA. Clear cell variant of squamous cell carcinoma of skin: A report of a case. J Oral Maxillofac Pathol. 2013;17:110-2.
5. Cohen PR, Schulze KE, Rady PL, Tyring SK, He Q, Martinelli PT, et al. Coincidental consort clear cell cutaneous carcinoma: Facial squamous cell carcinoma in situ containing human papillomavirus and cancer cells with clear cytoplasm in an octogenarian couple. South Med J. 2007;100:525-30.
6. Wang NR, Wang MM, Zhou L, Liu ZL, Chen NP, Hu JP, et al. Cutaneous clear cell/signet-ring cell squamous cell carcinoma arising in the right thigh of a patient with type 2 diabetes: combined morphologic, immunohistochemical, and etiologic analysis. Diagn Pathol. 2016;11:36.
7. Lee KH, Cho YK, Han YW, Kahng J, Park CJ. A case of clear-cell Bowen’s disease which may have developed following cryotherapy. Korean J Dermatol. 2005;43:1102-4.
8. Sezer E, Yuksek J. Warty and clear-cell Bowen’s disease successfully treated with photodynamic treatment. Photodermatol Photoimmunol Photomed. 2010;26:48-50.
9. Corbalán-Vélez R, Ruiz-Macia JA, Brufau C, López-Lozano JM, Martínez-Barba E, Carapeto FJ. Clear cells in cutaneous squamous cell carcinoma. Actas Dermosifiliogr. 2009;100:307-16.
10. Al-Arashi MY, Byers HR. Cutaneous clear cell squamous cell carcinoma in situ: clinical, histological and immunohistochemical characterization. J Cutan Pathol. 2007;34:226-33.
11. Biswas A, Mahalingam M. Cutaneous clear cell neoplasms: a histopathological reappraisal. Am J Dermatopathol. 2012;34:237-54.
12. Dalton SR, LeBoit PE. Squamous cell carcinoma with clear cells: how often is there evidence of trichilemmal differentiation? Am J Dermatopathol. 2008;30:333-9.
13. Misago N, Ackerman AB. New quandary: tricholemmal carcinoma? Dermatopathol Pract Conceptual. 1999;5:463-73.
Notes
Source of Support: Nil
Conflict of Interest: None declared.


Comments are closed.