Immunohistochemical evaluation of E-cadherin expression in basal cell carcinoma of the skin
Vladimír Bartoš1, Milada Kullová2
1Department of Pathology, Faculty Hospital in Žilina, V. Spanyola 43, Žilina, 012 07, Slovakia, 2Department of Dermatovenerology, Faculty Hospital in Žilina, V. Spanyola 43, Žilina, 012 07, Slovakia
ABSTRACT
Introduction: E-cadherin is important cell-cell adhesion molecule, that plays a crucial role in the maintenance of tissue microarchitecture. In many human malignancies, reduced or loss of E-cadherin production in neoplastic cells correlates with tumor dedifferentiation and acquisition of the invasive and metastatic potential. In contrast to most other cancers, basal cell carcinoma (BCC) of the skin possess some unique features, such as slow local growth, strong stroma-dependency, and virtual absence of metastases.
Aim: In the present study, we immunohistochemically evaluated expression of E-cadherin in a set of cutaneous BCCs.
Material and methods: Study group consisted of 41 primary BCCs cathegorized into non-infiltrative subroup (superfical and nodular subtypes) and infiltrative subroup (nodular-infiltrative and infiltrative subtypes).
Results: E-cadherin was expressed in all tumor specimens with variable quantitative range and intensity. There were 19 cases (46.3 %) with preserved and 22 cases (53.7 %) with reduced E-cadherin expression. In superficial, nodular, nodular-infiltrative and infiltrative BCC subtypes, reduced E-cadherin immunoreactivity was found in 40 % (2/5), 56.2 % (9/16), 54.5 % (6/11) and 55.5 % (5/9), respectively. We did not confirm a significant correlation between expression of E-cadherin and both given, non-infiltrative and infiltrative BCC subgroup. None of the tumors examined showed apparent decreasing immunostaining intensity in tumor tissue with increasing depth of invasion. There were not convincing differences either between the central and peripheral parts of tumor mass, or in the vertical dimension.
Conclusions: Reduction of E-cadherin expression per se does not seem to directly contribute to the acqusition of more aggressive phenotype in cutaneous BCC. This may represent another peculiarity, by which BCC differs from the most other epithelial malignancies and reflect a distinct tumor biology.
Key words: Basal cell carcinoma; E-cadherin; Immunohistochemistry
INTRODUCTION
Basal cell carcinoma (BCC) of the skin is histomorphologically and phenotypically very heterogeneous neoplasia. In contrast to most other cancers, it possess some unique features, such as slow local growth, strong stroma-dependency, and virtual absence of metastases [1–5]. Although mortality rates are very low, some BCCs may grow aggressively causing extensive tissue destruction and repeated recurrences after treatment [1–4]. Therefore, based on different biological behaviour and prognosis we distinct two BCC subgroups: Indolent subtypes (superficial and nodular), and aggressive subtypes (infiltrative, micronodular BCC and metatypical carcinoma) [1,3]. Recently, there is not definitively explained, whether they are a part of a continuous spectrum of tumorigenesis, starting with indolent and ending with aggressive forms, or they represent separate developmental lines [6].
Untill now, various biomarkers have been identified in cutaneous BCC involved in the mechanisms of cancer evolution and progression, some of which have or could have a great importance in predicting further clinical outcome [6]. Among them, E-cadherin has been studied in several papers [7–12] giving diverse or contradictory conclusions. Cadherins comprise a large family of transmembrane or membrane-associated calcium-dependent glycoproteins that mediate specific cell-cell adhesion and function as a key molecule in the histogenesis of various organs. They play a crucial role during embryogenesis and morphogenesis, as well as in the maintenance of adult tissue microarchitecture [14]. Intracellulary, they interact with several proteins, collectively termed catenins, which link them to the actin-based cytoskeleton. The cadherin family consists of at least five major subfamilies, i.e. “classical” cadherins of type I (including the best known epithelial E- cadherin, neural N-cadherin and placental P-cadherin), closely related cadherins of type II, desmosomal cadherins, protocadherins, and a variety of cadherin-related molecules [13]. The prototype of all cadherins is generally considered E-cadherin because it belongs to the most important molecules in cell-cell adhesion in epithelial tissue and has probably been studied in most detail, both in normal and pathological conditions [13,14].
The human E-cadherin gene CDH1 plays (besides other functions) a major role in tumor development and progression [14]. The suppression of E-cadherin production is regarded as one of the main molecular events responsible for dysfunction in cellular adhesion and tissue integrity, that help in local tumor invasion. Therefore, loss of function of E-cadherin or inactivation of cadherin-catenin complex correlates with dedifferentiation and acquisition of the invasive and metastatic potential of tumor cells, resulting in it being referred to as the “suppressor of invasion” gene [14]. In biopsy specimens, reduced or loss of E-cadherin expression correlates with epithelial cancer cells dedifferentiation and was found as an indicator of unfavourbale prognosis in many human malignancies, for example in oral squamous cell carcinoma [15], oesophageal carcinoma [16], carcinoma of the breast [17], ovarian carcinoma [18], pancreatic adenocarcinoma [19], rectal carcinoma [20], or non-small cell lung cancers [21]. Thus, these observations suggest that E-cadherin is implicated in the acquisition of invasive and metastatic potential of human cancer cells. Among skin malignancies, reduced expression of E-cadherin has been well documented in squamous cell carcinoma [22,23]. However, in cutaneous BCC, although it is the most common malignancy in humans, this relationship is still unclear. As mentioned above, several studies have been published to date dealing with this issue in human BCC, some of which provided conflicting results. Whereas some authors [9–12] demonstrated that expression of E-cadherin in BCC cells was quite frequently reduced and its decrease or loss was associated with more aggressive tumor biology, the results of other studies [7,8] did not confirm such assumption. Therefore, we focused on immunohistochemical analysis of E-cadherin expression in BCC to elucidate these discrepancies and to throw light on its potential role in the pathogenesis of this cancer.
MATERIAL AND METHODS
Tissue Specimens
Biopsy samples from 41 chosen cases of cutaneous BCCs of different histological types from various anatomical locations were enrolled into this study. They were obtained from 33 subjects (22 men, 11 women) in the age range 35 – 91 years (mean age 73.4 years). Topographical distribution of the tumors was as follows: head and neck (n = 32), trunk (n = 5), and extremity (n = 4). All patients were treated at the clinical departments of the Faculty Hospital in Zilina (Slovakia) and biopsy specimens were histopathologically investigated at the Department of Pathology in Faculty Hospital in Zilina during year 2014. For the purpose of this study, we deliberately selected a set of representative samples of cutaneous BCCs included four basic histomorphological subtypes: superficial, nodular, mixed nodular-infiltrative and infiltrative. We aimed to achieve two separate subgroups comprising roughly the same number of tumors. Thus, the first subgroup consisted of 21 indolent (non-infiltrative) BCC subtypes (5 superficial, 16 nodular) characterized by expansive growth pattern with clearly visible peripheral palisading. The second subgroup consisted of 20 BCCs with (at least focal) infiltrative growth pattern (11 nodular-infiltrative, 9 infiltrative subtypes), which was characterized by strands, cords or small separate tumor clusters without peripheral palisading, that invaded adjacent stroma. Metatypical BCC cases were exluded due to their intermediate features with squamous cell carcinoma. Only samples with enough tumor tissue in the paraffin-embedded blocks to harvest appropriate slides for immunohistochemistry were chosen.
Immunohistochemistry
Tissue specimens were routinely processed and immunohistochemical stained for E-cadherin. Briefly, representative 4-µm tissue sections applied on silanized slides were baked for 2 hours in an oven at 56 °C. Then the sections were deparaffinized in xylene for 2 × 15 minutes, rehydrated in series of descending ethanol concentrations and treated with microwaves in a 0.01 M citrate buffer (pH 6.0) for 15 minutes. The endogenous peroxidase activity was blocked with 3 % hydrogen peroxide for 10 minutes, followed by incubation with TBS (tris-buffered saline) solution (pH 7.6). Subsequently, specific monoclonal mouse anti-human antibody against E-cadherin (clone NCH-38, code M3612, DAKO, dilution 1: 50) was used for staining. After overnight incubation at ambient temperature, post primary antibody (rabbit anti mouse IgG and anti rabbit Poly-HRP-Ig containing 10 % animal serum in TBS, Leica Biosystems) was applied and an immunoreaction was visualised by means of the DAB (3,3’-diaminobenzidine) detection chromogen solution (Leica Biosystems) according to manufacturer’s instructions. Slides were counterstained with Weigert’s hematoxylin, dehydrated, mounted and finally evaluated in the light microscope.
Immunohistochemical Interpretation
According to study previously published by Pizzaro et al. [9] we classified the intensity of immunostaining in tumour cells as (+ +) when as strong as the normal epidermis, (+) when weak, and (-) when cells were not stained. After including a total percentage of immunolabelled tumor cells, as proposed Pizzaro et al. [9] we differed following four cathegories: a) BCC with preserved E-cadherin expression (more than 75% of the tumour cells were strongly (+ +) stained), b) BCC with slightly reduced E-cadherin expression (more than 25% of the tumour cells were positively stained but less than 75% of the tumour cells were strongly (+ +) stained), c) BCC with severely reduced E-cadherin expression (more than 75% of tumor cells were not stained), and finally d) BCC with absent E-cadherin expression (immunostaining was completely lost). In addition, we qualitativelly differed homogeneous and heterogeneous pattern of E-cadherin immunoreactivity. Homogeneous pattern was characterized by virtually the same staining intensity (regardless strong or weak) throughout the whole immunolabeled tumor tissue. In heterogeneous pattern, tumor areas with variable intensity of the immunolabeled cells (strong versus weak) were clearly visible.
Statistical Analysis
Data were collected in a databank, using a software SPSS Statistics. For the statistical analysis, chi-square test was employed and P value < 0.05 was considered to indicate statistical significance.
Ethics
This study was performed on human subjects; thus, all patients were aware of the presence of the study and they were fully informed about the drug and its side-effects.
RESULTS
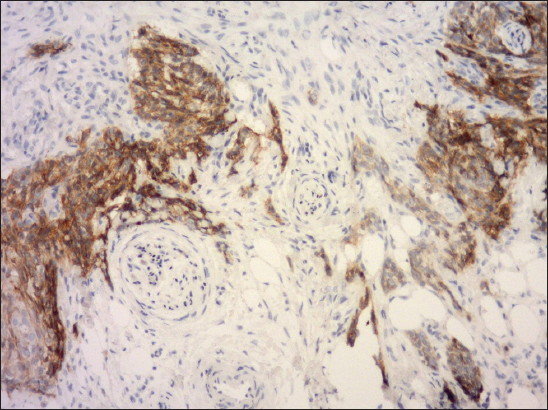
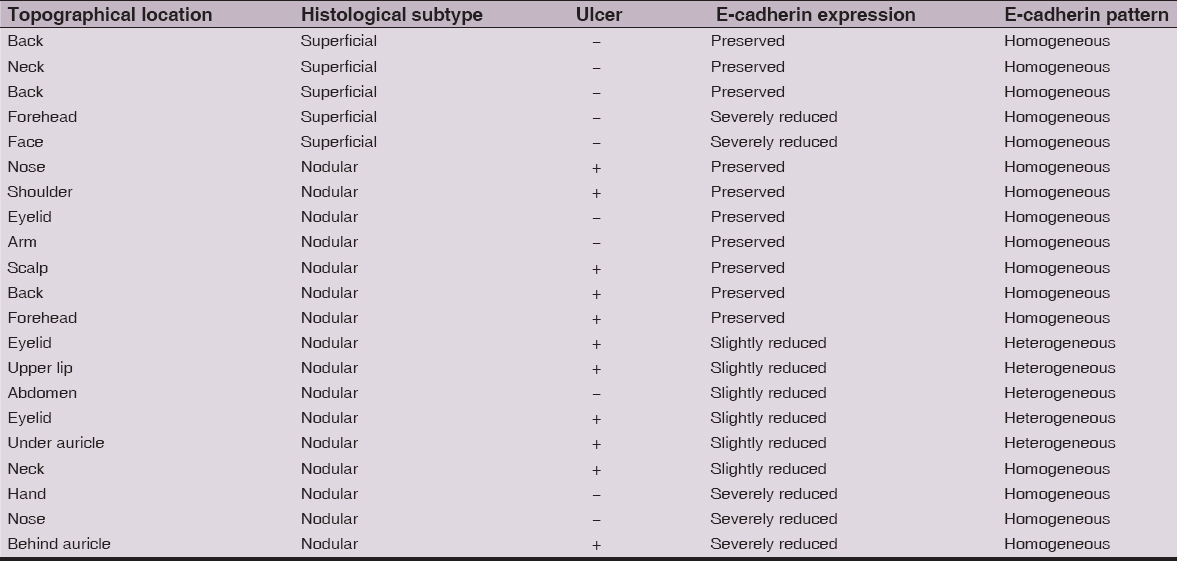
E-cadherin was expressed in all tumor specimens with variable quantitative range and intensity showing a linear pattern around the periphery of the cancer cells. A faint diffuse cytoplasmic staining was also observed in a minority of cases. No nucler immunoreactivity was detected. Overall, there were 19 cases (46.3%) with preserved (Fig. 1) and 22 cases (53.7%) with reduced E-cadherin expression, of which 15 cases were slightly and 7 cases severely reduced. None of the tumors investigated showed a completely negative staining. In superficial, nodular, mixed nodular-infiltrative and infiltrative BCC subtypes, reduced E-cadherin immunoreactivity was found in 40% (2/5), 56.2% (9/16), 54.5% (6/11) and 55.5% (5/9), respectively. Among them, severely reduced expression (< 25 % of entire tumor tissue) was seen in 40% of superficial BCCs (2/5), in 18.7% of nodular BCCs (3/16), in 9.1% of nodular-infiltrative BCCs (1/11), and 14.2% of infiltrative BCCs (1/7) (Fig. 2). We did not confirm a statistically significant correlation between immunohistochemical expression of E-cadherin (preserved versus reduced) and both given, non-infiltrative and (at least focally) infiltrative BCC subgroup (p = 0.8). In general, among four histological subtypes, diminished E-cadherin expression occured most commonly in nodular-infiltrative BCCs. However, when we precisely analyzed microarchitecture in all 11 cases we have found that in both structural components, virtually the same immunostaining intensity was seen in eight cases (72.7%) (Fig. 3). Only one tumor showed a weaker immunoreactivity in the infiltrative component and surprisingly, in the remaining two cases, tumor areas with infiltrative growth seemed to have a stronger immunopositivity compared to nodular component. As for spatial distribution, indolent (non-infiltrative) BCC subgroup usually showed a widely homogeneous staining positivity (16/21, 76.1%) throughout the tumor mass regardless of whether preserved or reduced E-cadherin expression. On the other hand, in infiltrative BCC subroup, a heterogeneous staining positivity (16/20, 80.0%) was more commonly seen. We confirmed a significant correlation between immunoreactivity pattern of E-cadherin and tumor growth microarchitecture in terms of more frequent heterogeneous pattern in BCCs with infiltrative growth features (p < 0.003). Further, while homogeneous pattern was more commonly associated with membranous E-cadherin positivity solely (18/20, 90%), heterogeneous pattern was mostly associated with mixed cytoplasmic-membranous positivity (15/21, 71.4%). In spite of this qualitative diversity of E-cadherin expression, none of the 41 cacinomas examined manifested apparent decreasing staining intensity in tumor tissue with increasing depth of invasion. Although BCCs having heterogeneous E-cadherin pattern exhibited mixed, weak and strong positive areas, as well as population of negative cells intermingled with clusters of positive cells, there were not convincing differences either between the central and peripheral parts of tumor mass, or in the vertical dimension. Interestingly, some BCCs belonging to infiltrative subgroup showed much more pronounced E-cadherin expression at the invasive fronts of tumor mass, which invaded deeply into the corium or subcutis (Fig. 4). Moreover, one of them also exhibited a multiple perineural tumorous infiltration (Fig. 5), which is a sign of aggressive tumor behaviour. There was not found a statistical correlation between E-cadherin expression and tumor ulceration (p = 0.1) or gender (p = 0.7). A summary of the morphological characteristics and immunohistochemical findings in our set of BCCs divided into two subgroups described above is presented in Table 1 and 2.
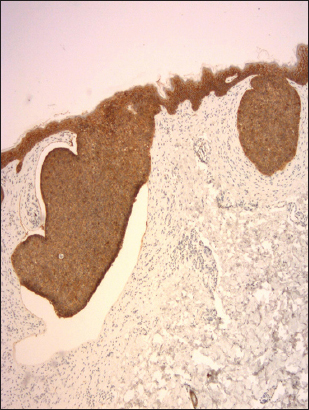
Figure 1: Preserved expression of E-cadherin with homogeneous staining pattern in superficial BCC (clone NCH-38, DAKO, original magnification 120×).
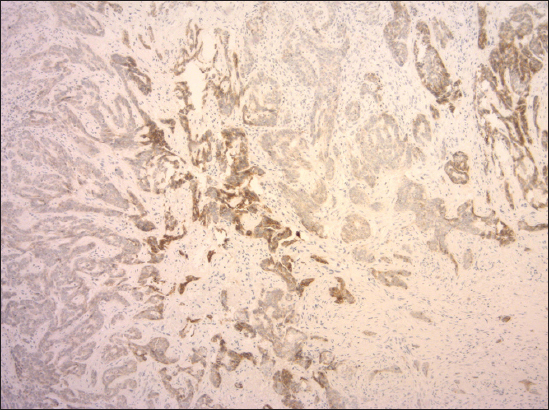
Figure 2: Severely reduced expression of E-cadherin with heterogeneous staining pattern in infiltrative BCC. Some parts of tumor are completely negative (clone NCH-38, DAKO, original magnification 120×).
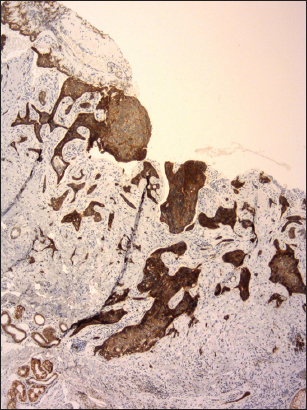
Figure 3: Preserved expression of E-cadherin with heterogeneous staining pattern in mixed nodular-infiltrative BCC (clone NCH-38, DAKO, original magnification 120×).
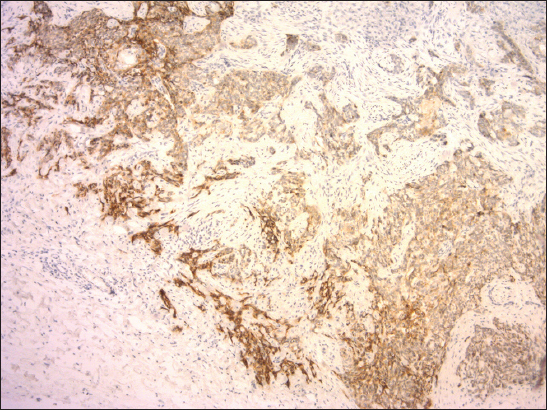
Figure 4: Slightly reduced expression of E-cadherin with heterogeneous staining pattern in mixed nodular-infiltrative BCC. Invasive front of tumor mass (left) consisting of infiltrative component exhibited stronger immunopositivity compared to nodular component (right) (clone NCH-38, DAKO, original magnification 120x).
Figure 5: Predominantly strong expression of E-cadherin in the invasive edges of infiltrative BCC, that grow deeply into subcutaneous tissue and exhibit perineural tumor infiltration (clone NCH-38, DAKO, original magnification 240×).
Table 1: The morphological and immunohistochemical findings of 20 tumors included in the infiltrative BCC subgroup (Ulcer − tumor ulceration, + present, − absent)
Table 2: The morphological and immunohistochemical findings of 21 tumors included in the indolent (non-infiltrative) BCC subgroup (Ulcer − tumor ulceration, + present, − absent)
DISCUSSION
This paper describes immunohistochemical expression status of cell-cell adhesion molecule E-cadherin in a panel of 41 human BCCs of the skin. As pointed above, we found reduced E-cadherin expression in 53.7% cases investigated, indicating a relatively common phenomenon. More importantly, we did not shown significant association of decreasing production of E-cadherin with more aggressive tumor growth. It should be noted, however, conflicting views have been reported on the role of E-cadherin status in BCC carcinogenesis and biological behaviour until now. For example, some authors [7,8] immunohistochemically analysed E-cadherin in cutaneous BCCs and found, it was strongly expressed in most cases examined. From the results of their studies they suggested, virtually no metastatic potential of cutaneous BCC may be due to retention of high levels of E-cadherin production in tumor cells.
However, another group of authors claimed [9–12] that intensity of E-cadherin expression in BCC is variable and depends on tumor histomorphology, being weaker or even absent in the infiltrative (morpheaform) subtypes. Two decades ago Pizzaro et al. [9] analysed 31 cutaneous BCCs and found, E-cadherin expression was preserved in all specimens of superficial and nodular BCC, but 66.6 % of the infiltrative BCCs showed decreased expression. Statistical analysis showed a significant association between reduction in E-cadherin expression and the infiltrative growth pattern. Based on these observations they stated, E-cadherin status may contribute to the growth pattern and the local aggressive behaviour of BCC.
Remarkably, none of the tumors in their series showed completely negative immunostaining. Therefore, there are quite surprising results in a recent work published by Papanikolau et al. [10] who observed completely absent membranous E-cadherin immunoreactivity in 71 out of 100 BCC cases. In addition, they recorded a preserved membranous E-cadherin expression only in 8% of all cases examined. Nevertheless, a decrease in membranous E-cadherin expression correlated significantly with infiltrative BCC variant, as well as depth of tumor invasion. Additional controversial results are given by Uzquiano et al. [8] who analysed a set of 32 cutaneous BCCs including 12 nodular subtypes, 10 infiltrative subtypes, and 10 metastatic forms. They found E-cadherin expression in almost the same proportion of nodular (66. %) and infiltrative BCCs (70%). However, it was demonstrated in all (100%) metastatic BCCs, of which all but one exhibited infiltrative growth features. There was a statistically significant difference in expression of E-cadherin between metastatic BCC as compared to nodular BCC, being increased in metastases. These observations are interesting and call into question a relationship between decline of E-cadherin production in BCC cells and acquisition of more aggressive tumor biology.
The results of our study suggest that aggressive growth features of BCC are not directly associated with a decrease of E-cadherin production in epithelial tumor cells. This view is supported by several facts. Firstly, reduced E-cadherin expression was observed in similar percentage of all BCC subtypes investigated, moreover, without a statistical significance between non-infiltrative and (at least partially) infiltrative growth variants. Secondly, there was observed no “vertical gradient” of decreasing staining intensity in cancer tissue with increasing depth of invasion. Thirdly, in most cases of mixed nodular-infiltrative BCCs, both structural components exhibited about the same immunostaining intensity. Finally, cases accompanied by severely reduced E-cadherin expression occured most frequently in superficial and nodular BCC, while they were uncommon in pure infiltrative BCC subtype. Therefore, we are of the opinion, decrease or loss of production of this cell-cell adhesion molecule per se is not directly implicated in the acquisition of invasive potential of BCC cancer cells. More intriguingly, we showed a correlation between pattern of E-cadherin immunoreactivity and tumor growth microarchitecture, being homogeneous more frequently in non-infiltrative subtypes and heterogeneous predominantly in BCCs with infiltrative growth features. This may indicate that qualitative aspects of E-cadherin expression have a greater importance in biological behaviour of cutaneous BCC and should be taken into account at the histopathological examination and biopsy report interpretation. For instance, such relationship has already been revealed in carcinoma of the rectum. Kanazawa et al. [20] studied 43 primary rectal cancers and found that heterogeneous E-cadherin immunostaining pattern, when compared to homogeneous one, was significantly associated with poorer differentiation of tumors and a presence of lymph node metastases. Therefore, a presence of tumor areas with notably different E-cadherin expression may suggest a cancer progression and evolution of polyclonal neoplastic cell population with unstable genotype and thus, accompanied by higher tendency for more aggressive growth. In practical terms, nodular cutaneous BCC with severely reduced E-cadherin expression, however, with homogeneous pattern of immunolabelled tumor cells would not have such aggressive biological behaviour as infiltrative BCC with only slightly reduced expression, but heterogeneous staining.
One limitation of our study is a relatively small number of cases investigated. Anyway, this number is larger than those published by Pizzaro et al. [9] and Uzquiano et al. [8] who found their observations statistically relevant. We can assume that the discrepancy with the results among individual authors may be related, at least in part, to the different proportion of BCC subtypes included in their series. The controversies may be also due to the distinct anatomic location of tumors examined, since it can not be excluded that some topographic-related factors, for example intensity of solar exposition, may influence pathogenetic mechanisms and consequently molecular phenotype of BCCs.
Recently, there is growing evidence that invasiveness and tendency of more aggressive BCC growth are largely influenced and modified by adjacent peritumorous stroma [5,24]. Some authors [5] identified a specific molecular profile of fibroblasts isolated from the surrounding peritumorous tissue in human BCCs when compared to those extracted from other epithelial malignancies. These results suggest unique molecular phenotype of cancer-associated fibroblasts in BCC possibly accounting for diseae-specific pathological roles including stroma-dependency and “non-metastatic” behaviour. In addition, BCC cells alone exhibited some mesenchymal markers with contractile properties, such as α-smooth muscle actin or calponin [8,25], which are unsusual for epithelial origin and are considered to be specifically involved in the epithelial-mesenchymal transition and cancer progression.
In conclusion, E-cadherin expression in BCC of the skin is variable and its decline within tumor cells is a relatively frequent finding. There is no doubt, this important cell-cell adhesive molecule is implicated in BCC development and carcinogenesis. However, reduction of E-cadherin expressionper se does not seem to directly contribute to the acqusition of more aggressive phenotype in cutaneous BCC. This may represent another peculiarity, by which BCC differs from the most other epithelial malignancies and reflect a distinct tumor biology.
ACKNOWLEDGMENTS
The authors would like to thank Mrs. Hanajíkova Tat’ana and MUDr. Doboszová Jana for their outstanding educational and technical assistance.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent
Informed consent was obtained from all patients for being included in the study.
REFERENCES
1. Bartoš V, Adamicová K, Kullová M, Péč M, Immunohistochemical evaluation of proliferative activity (Ki-67 index) in different histological types of cutaneous basal cell carcinomaBiologia 2012; 67: 610-5.
2. Abdulaziz A, Brzezinski P, Chiriac A, Skin of sailor: cutis rhomboidalis nuchae, actinic keratosis, squamous cell carcinoma and basal cell carcinoma. Case reportOncoReview 2014; 4: A36-40.
3. Crowson AN, Basal cell carcinoma: biology, morphology and clinical implicationsMod Pathol 2006; 19: S127-S7.
4. Brzezinski P, Andruszkiewicz J, Sinjab AT, Chiriac A, Recurrent basal cell carcinoma in patient treated by cryotherapyJPAD 2013; 23: 436-9.
5. Micke P, Kappert K, Ohsima M, Sundquist C, Scheidl S, Lindahl P, In situ identification of genes regulated specifically in fibroblasts of human basal cell carcinomaJ Investig Dermatol 2007; 127: 1516-23.
6. Bartoš V, Adamicová K, Kullová M, Péč M, Basal cell carcinoma of the skin – biological hehaviour of the tumor and a review of the most important molecular predictors of disease progression in pathological practiceKlin Onkol 2011; 24: 8-17.
7. Bozdogan O, Yulug IG, Vargel I, Cavusoglu T, Karabulut AA, Karahan G, Sayar N, Differential expression patterns of metastasis suppressor proteins in basal cell carcinomaInt J Dermatol 2014; Nov27doi: 10.1111/ijd.12581. [Epub ahead of print]
8. Uzquiano MC, Prieto VG, Nash JW, Ivan DS, Gong Y, Lazar AJ, Metastatic basal cell carcinoma exhibits reduced actin expressionMod Pathol 2008; 21: 540-43.
9. Pizzaro A, Benito N, Navarro P, Palacios J, Cano A, Quintanilla M, E-cadherin expression in basal cell carcinomaBr J Cancer 1994; 69: 157-62.
10. Papanikolaou S, Bravou V, Gyftopoulos K, Nakas D, Repanti M, Papadaki H, ILK expression in human basal cell carcinoma correlates with epithelial-mesenchymal transition markers and tumour invasionHistopathology 2010; 56: 799-809.
11. Vanjaka-Rogošić L, Puizina-Ivić N, Mirić L, Rogošić V, Kuzmić-Prusac I, Babić MS, Matrix metalloproteinases and E-cadherin immunoreactivity in different basal cell carcinoma histological typesActa Histochem 2014; 116: 688-93.
12. Tucci MG, Lucarini G, Zizzi A, Rocchetti R, Brancorsini D, Di Primio R, Cdc42 is involved in basal cell carcinoma carcinogenesisArch Dermatol Res 2013; 305: 835-40.
13. Van Roy F, Berx G, The cell-cell adhesion molecule E-cadherinCell Mol Life Sci 2008; 23: 3756-88.
14. Pećina-Slaus N, Tumor suppressor gene E-cadherin and its role in normal and malignant cellsCancer Cell Int 2003; 3: 17
15. Liu LK, Jiang XY, Zhou XX, Wang DM, Song XL, Jiang HB, Upregulation of vimentin and aberrant expression of E-cadherin/β-catenin complex in oral squamous cell carcinomas: correlation with the clinicopathological features and patient outcomeMod Pathol 2010; 23: 213-24.
16. Xu XL, Ling ZQ, Chen SZ, Li B, Ji WH, Mao WM, The impact of E-cadherin expression on the prognosis of esophageal cancer: a meta-analysisDis Esophagus 2014; 27: 79-86.
17. ElMoneim HM, Zaghloul NM, Expression of e-cadherin, n-cadherin and snail and their correlation with clinicopathological variants: an immunohistochemical study of 132 invasive ductal breast carcinomas in EgyptClinics (Sao Paulo) 2011; 66: 1765-71.
18. Taşkin S, Dünder I, Erol E, Taşkin EA, Kiremitçi S, Öztuna D, Sertçelik A, Roles of E-cadherin and cyclooxygenase enzymes in predicting different survival patterns of optimally cytoreduced serous ovarian cancer patientsAsian Pac J Cancer Prev 2012; 13: 5715-9.
19. Hong SM, Li A, Olino K, Herman JM, Schulick RD, Iacobuzio-Donahue C, Loss of E-cadherin expression and outcome among patients with resectable pancreatic adenocarcinomasMod Pathol 2011; 24: 1237-47.
20. Kanazawa N, Oda T, Gunji N, Nozue M, Kawamoto T, Todoroki T, E-cadherin expression in the primary tumors and metastatic lymph nodes of poorly differentiated types of rectal cancerSurg Today 2002; 32: 123-8.
21. Yan B, Zhang W, Jiang LY, Qin WX, Wang X, Reduced E-Cadherin expression is a prognostic biomarker of non-small cell lung cancer: a meta-analysis based on 2395 subjectsInt J KClin Exp Med 2014; 7: 4352-6.
22. Lyakhovitsky A, Barzilai A, Fogel M, Trau H, Huszar M, Expression of e-cadherin and beta-catenin in cutaneous squamous cell carcinoma and its precursorsAm J Dermatopathol 2004; 26: 372-8.
23. Li ZX, Peng ZH, Ji FP, Yuan JY, Pan M, Liu Y, Expression of E-cadherin and beta-catenin in Bowen’s disease and squamous cell carcinomaNan Fang Yi Ke Da Xue Xue Bao 2006; 26: 1245-7.
24. Marsh D, Dickinson S, Neill GW, Marshall JF, Hart IR, Thomas GJ, Alpha vbeta 6 Integrin promotes the invasion of morphoeic basal cell carcinoma through stromal modulationCancer Res 2008; 68: 3295-303.
25. Lee MW, Ahn SJ, Choi JH, Moon KC, Koh JK, Actin and calponin expression in basal cell carcinomaBr J Dermatol 2004; 151: 934-6.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.

Comments are closed.