Age-related differences in the incidence and clinicopathological findings of malignant melanoma of the skin
Vladimír Bartoš1, Milada Kullová2
1Department of Pathology, Faculty Hospital in Žilina, V. Spanyola 43, Žilina, 012 07, Slovakia, 2Department of Dermatovenerology, Faculty Hospital in Žilina, V. Spanyola 43, Žilina, 012 07, Slovakia
ABSTRACT
Introduction: In malignant melanoma of the skin, age has been found to be one of the factors associated with different clinical outcome and prognosis of disease. The purpose of our study was to investigate differences in the incidence and clinicopathological findings of cutaneous melanoma in relation to the age of the patients.
Material and Methods: Study group consisted of 116 primary invasive malignant melanomas of the skin from 116 subjects (57 men, 59 women) between 31 – 93 years of age. They were stratified into three separate age groups: group I (≤ 43 years, young age), group II (44 – 60 years, middle age) and group III (≥ 61 years, old age).
Results: In group I, II and III, we confirmed 22 (19%), 45 (38.8%) and 49 (42.2%) individuals, respectively. As age increased, proportion of males, as well as melanomas located on the head and neck were rising. There was evident decline in the percentage of superficial spreading melanoma and conversely, increase in the percentage of nodular melanoma. Acral lentiginous melanoma and lentigo maligna melanoma was found only in patients over 60 years old. In general, advancing age was associated with lower prevalence of stage pT1 and higher prevalence of stage pT4, higher mean Breslow´s thickness and mitotic rate and with larger proportion of ulcerated lesions. In comparision to younger individuals, patients ≥ 61 years of age exhibited melanocytic nevus remnants in melanomas less commonly.
Conclusion: We found apparent age-related differences in the incidence and clinicopathological characteristics of malignant melanoma of the skin. In general, unfavourable prognostic variables predominated in the oldest population. Further research is needed to clarify, to what extent are these age-related disparities associated with distinct etiopathogenetic mechanisms and primary tumor biology.
Key words: Malignant melanoma; Age; Prognosis
INTRODUCTION
The incidence of malignant melanoma of the skin has dramatically increased over the last several decades [1–3]. Although some recent data indicate that the rate of such increase began to stabilize, this has been observed predominanly in younger perons [2]. Conversely, the rate of increase in both, incidence and mortality has been significantly higher for age groups older than 60 years [2]. In general, malignant melanoma is one of the most aggressive neoplasms in humans characterized by a relatively poor prognosis. In developed countries, it represents the leading cause of death among malignant skin tumors [3]. However, overall clinical outcome of disease directly depends on several clinical and histopathological parameters [4], which individually determine the choice of the therapeutic strategies and managment of the patients. Among them, age has been found to be one of the clinical factors associated with different incidence, clinical outcome and overall prognosis of disease. Many studies demonstrated [1,2,5–11], that clinical presentation and pathological characteristics in melanoma of the elderly differ from that of their younger counterparts. When compared to younger age groups, older individuals are more likely to acquire and to die from melanoma implicating age as a poor prognostic factors [12]. The most striking differences in melanoma incidence and mortality occur in individuals over age 65, although modest differences are notable in those over age 50 [12]. We undertook a retrospective study to investigate the differences in the incidence and clinico-pathological prognostic factors of cutaneous melanoma in relation to the age of the patients.
MATERIAL AND METHODS
Study group consisted of 116 consecutive representative primary invasive malignant melanomas of the skin, that were histologically diagnosed at the Department of Pathology in Faculty Hospital in Zilina (Slovakia) between January 2007 – October 2014. They were obtained from 116 subjects (57 men, 58 women) in the age range between 31 – 93 years. In situ and reccurent melanomas, as well as mucosal and ocular melanomas were excluded from the analysis. The topographic localization of tumors studied was as follows: head and neck (n = 12), trunk (n = 56), upper extremities (n = 32) and lower extremities (n = 16). The lesions were excised in several clinical departments of the hospital (i.e., departments of surgery, dermatovenerology and otorhinolaryngology). Treatment consisted of wide surgical extirpation and a subsequent regional lymph node disection was performed in some patients (n = 13) with suspected regional metastases. All tumors were removed completely with negative surgical margins and objective assessment of all conventional histomorfological parameters was possible. In each case, melanoma characteristics that were analyzed for the purpose of this study included: histologic type, tumor thickness according to Breslow, level of invasion as defined by Clark, pT-stage, ulceration, pre-existing nevus, mitotic rate expressed as the number of mitoses per square millimeter and sentinel lymph node status. All cases were staged according to 7th edition of UICC (Union for International Cancer Control) TNM classification of tumors [13]. Biopsy material was fixed in buffered formalin, embedded in paraffin blocks, stained with hematoxylin and eosin, and the slides were reviewed by pathologists under the light microscope. In addition to standard hematoxylin and eosin staining, we also use special histochemical (Pearls, Fontana-Masson stains) and immunohistochemical (antibodies against melan A, HMB-45, S-100 protein, polyclonal cytokeratins, Ki-67 antigen) methods for better microscopic evaluation of tumor tissue. According to recent paper published by Liljana Mervic [14], patients were cathegorized into three separate age groups: group I (≤ 43 years, young age), group II (44 – 60 years, middle age) and group III (≥ 61 years, old age). After this stratification, we compared clinicopathological data between all given groups. Informations on patients were received from the clinical hospital records, or by consultation with the clinicians.
RESULTS
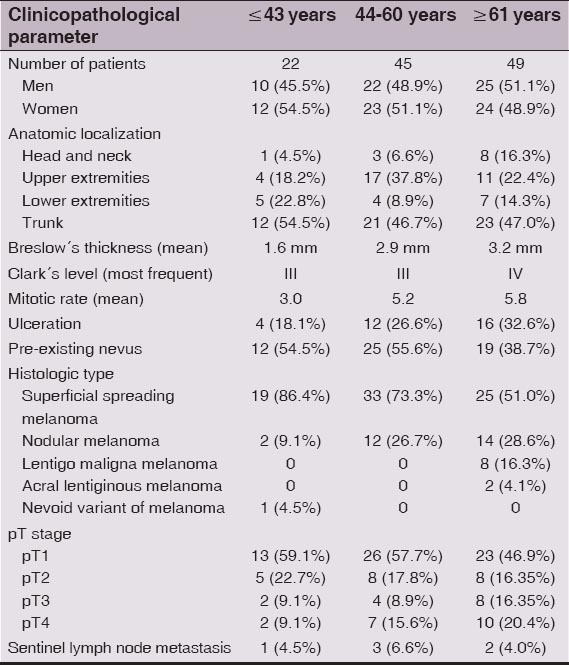
A summary of the distribution of various clinical and histopathological characteristics of our cohort of patients stratified by age groups is presented in Table 1. Overall, group I, II and III consisted of 22 (19.0%), 45 (38.8%) and 49 (42.2%) individuals, respectively. The mean age of the patients at the time of primary diagnosis was 58.4 years. As age increased (in terms of group I → II → III), there was a mild increase in the prevalence of men and decrease in the prevalence of women. While male/female ratio was 1.2 in the youngest group, it was only 0.9 in patients older than 60 years. In each age group, melanomas occured most frequently on the trunk, especially on the back. However, advancing age was associated with rising percentage of tumors located on the head and neck. Whereas they represented only 4.5% of all melanomas diagnosed in group I, it was more than 3 times (16.3%) in group III. Conversely, in the youngest group there was a higher prevalence of melanomas located on the trunk (54.5%), although when compared to both other groups (46.7% and 47%), these differences were not so much striking. In addition, as age increased, there was continuous decline in the percentage of superficial spreading melanoma (86.4% vs 73.3% vs 51%) and increase in the percentage of nodular melanoma (9.1% vs 26.7% vs 28.6%). Acral lentiginous melanoma and lentigo maligna melanoma were found only in patients over 60 years old. Comparative analysis of three given age groups have shown that advancing age was associated with lower prevalence of stage pT1 (59.1% vs 57.7% vs 46.9%) and higher prevalence of stage pT4 (9.1% vs 15.6% vs 20.5%), greater mean tumor thickness (1.6 mm vs 2.9 mm vs 3.2 mm) as well as number of mitoses per 1 mm2 (3.0 vs 5.2 vs 5.8) and with larger proportion of ulcerated lesions (18.1% vs 26.6% vs 32.6%). The elderly aged 60 and over were less likely to have melanoma histologically associated with a pre-existing melanocytic nevus (38.7%) compared to both younger groups, where this percentage was about the same (54.5% and 55.6%). We did not confirm convincing differences in the incidence of sentinel lymph node metastasis among the study age groups (4.5% vs 6.6% vs 4.0%), although this assessment can be considered only informative, since it is limited due to the small number of persons who underwent regional lymph node extirpation. Of these, the interval between histological verification of primary melanoma and lymph node metastasis varied from 1 to 18 months except for one patient, in whom both, skin lesion and positive lymph node biopsy were made during one surgical procedure.
DISCUSSION
Malignant melanoma of the skin exhibits a variety of clinicopathological differences depending on age, for example in the site distribution, gender prevalence, histomorphological features and prognostic variables derived therefrom. The results of our observations correspond to the literature data reporting that with increasing age, melanomas occur more frequently in men [1,2,5] and their proportion on the head and neck is rising [1,2]. Higher incidence of head and neck melanoma is likely attributable to cumulative photodamage as patients age [1]. While in young individuals a melanoma genesis is associated with intensive intermittent solar exposure and sunburns, in older people melanomas develop more commonly on the skin permanently exposed to sunlight throughout life [7]. Consequently, melanomas are more likely to arise on the trunk at a younger age, and on the head and neck in the elderly. From the practical point of view, as the percentage of older persons in the world’s population continues to grow, physicians performing melanoma screening should devote heightened attention to heavily sun-exposed body sites [1]. As for histological types, older patients are characterized by relatively higher percentage of lentigo maligna melanoma, nodular melanoma and acral lentiginous melanoma [2,7,11]. Our results are consistent with these reported data, as the highest percentage of nodular melanoma was present in patients over 60 years old and both, acral lentiginous melanoma and lentigo maligna melanoma were found only in this age cathegory. Melanoma in the elderly less likely arise from a pre-existing melanocytic nevus [1,15,16], but it is closely linked with their predominant histological types. Superficial spreading melanoma (which occur most commonly in younger persons and on the trunk) develops from previous melanocytic nevus most frequently [15,16]. On the contrary, lentigo maligna melanoma (which occur predominantly in the elderly and on the chronically sun-exposed body sites) has the lowest probability to be in continuity with associated nevus remnants [15,16]. Lentigo maligna melanoma possesses clinical characteristics and epidemiologic patterns that are distinct from other melanomas. As mentioned above, it usually develop later in life and has the strongest relationship with a chronic ultraviolet radiation among all types. These observations could suggest that lentigo maligna melanoma arises along a causal pathway driven by accumulated sun exposure as distinct from arising in association with a melanocytic nevus [15]. The vast majority of studies have shown, as we did, as age increased, percentage of thicker melanomas was significantly rising [1,2,5–11]. This is probably due to delayed diagnosis of disease, feature more typical for the elderly males [7], as well as apparent rising predponderance of nodular melanoma [2,10,17]. The significance of different melanoma subtypes is controversial in this regard, since the thickness of melanoma shows progressive diminution in nodular vs superficial spreading vs lentigo maligna melanoma [4]. However, it may reflect reproducible differences in the biology. Thus, nodular melanoma, which manifests a vertical growth phase de novo would naturally be expected to have the greatest thickness, however, also to contain a clone of neoplastic cells more capable of metastatic progression [4]. One study indeed found [18] that nodular melanoma, along with older age and male gender are associated with rapid and more aggressive tumor growth. Advancing age is also associated with greater percentage of tumor ulceration [2,5,8,9,11], tumor regression [5] and pronounced mitotic activity [1] – factors that reflect more aggressive biological behaviour of melanoma and adversely affect both, recurrence and mortality rate.
The issue of whether age alone directly correlates with clinical course and worse survival has been debated over the past several decades [12]. As most histopathological melanoma features worsen with increasing age, not surprisingly it parallels poorer prognosis. Therefore it has long been questionable whether age is really an independent significant prognosticator. However, the results of several multivariate studies have supported this assumption [2,6,8,9], since prognostic significance of age has been demonstrated after adjusting for all known prognostic variables. For example, Austin, et al [8] observed significant disease-free survival differences in the older population, with only 55% of the elderly population being disease free at 5 years compared with 65% for the younger population. Regression analysis showed age was an independent survival predictor. Lasithiotakis, et al [2] found, melanoma patients older than 65 years had lower 10-year disease-specific survival than younger ones and this difference was more pronounced in women than in men. Males had lower 10-year disease-specific survival than females but this difference did not reach statistical significance in individuals older than 65 years. In multivariate analysis, age was independent prognostic indicator. Similar results have also been reported by Balch, et al [6] and Caraco, et al [9]. This may suggest that the role of age in the natural course of melanoma might not be entirely explained by differences in the proportions of the known clinicopathologic variables of disease.
Unfavorable prognosis in older population may be explained by several reasons, such as diminished immune response with increased age, changes in host immune biology, decreased ability to repair DNA in sun-damaged melanocytes [12]. Older people don’t pay such attention to the pathological changes arising on their skin and they seek medical care in more advanced stage of disease [2,7,12]. Furthermore, worlwide educational and preventive programs for melanoma have generally targeted younger age groups [1,2]. The elderly have often (due to comorbidity) limited choice of the best therapeutic modality performation [2,12].
Cutaneous malignant melanoma has a clinical pecularity, which has not yet been completely elucidated and probably suggest different age-specific tumor biology. While increasing age is associated with worse prognosis, it is accompanied by significant decrease in the incidence of sentinel lymph node (SLN) metastasis. This inverse relationship (higher mortality rate and lower incidence of SLN metastasis in older people vs lower mortality rate and higher incidence of SLN metastasis in younger people) has been confirmed by many [1,2,5,19] but not all authors [9]. In the most recent robust study Balch et al [1,9] demonstrated, that the highest incidence of SLN metastasis (25.8%) was in melanoma patients younger than 20 years and the lowest (15.5%) among patients ≥ 80 years of age in spite of the fact, older persons had tumor features associated with more adverse histomorphology. While 5-year mortality was only 10% in individuals under 20 years of age, it reached 20% for those 20–40 years of age, 38% for those over 70 years of age and 45% for those age 80 years and older. These data confirm a phenotypic diversity of primary cutaneous melanomas, that should be taken into account in clinical practice. In addition, biological basis of this interesting phenomenon may be promising field for further research. It is quite possible, that skin melanomas in younger and older people have distinct genetic and molecular profile, different preferential routes of metastasis, as well as different host response to metastatisizing melanoma cells [19]. Indeed, relatively recently published study [20] has really demonstrated distinct expression of several genes (regulating for example cycle-cell mechanisms, inflammation, epithelial-mesenchymal transition) between the youngest and oldest patients with skin melanoma. Futhermore, there exist three main patterns of metastatic melanoma progression, which differ between men and women [14], a fact that may also be regarded age-related, as women are more commonly affected at a younger and men in older age.
As mentioned above, different types of primary melanoma with distinct prognosis are distributed unequally across age groups. A wide morphological diversity of malignant melanomas (and melanocytic lesions as general) is particularly observed among children and adolescents, in whom disease exhibited more favourable clinical outcome. One explanation may be, that very young people have more commonly diagnosed atypical spitzoid melanocytic tumors and melanocytic neoplasms of unknown malignant potential, both are known to have a better prognosis in comparision to “usual” cutaneous melanomas in spite of the fact, they are accompanied by regional lymph node metastasis more frequently [21,22]. Therefore, some authors [19] discuss about a possible inclusion of the patient´s age into the melanoma staging, as is already applied for example in follicular and papillary carcinoma of thyroid gland [13]. It is quite likely that cutaneous melanomas arising in the youngest and the oldest population have distinct etiopathogenesis and biological behaviour in comparision to “middle” age and hence, their clinical management and treatment should be performed differently.
CONCLUSION
We found apparent age-related differences in the incidence and clinicopathological characteristics of malignant melanoma of the skin. Since unfavourable prognostic variables generally predominated in the oldest group, the elderly comprise an important high-risk group among melanoma patients. We are of the opinion, further research is needed to clarify, to what extent are these age-related disparities associated with distinct etiopathogenetic mechanisms and primary tumor biology and how to translate the survival advantage of younger individuals into a survival benefit for all patients with cutaneous melanoma.
CONSENT
The examination of the patient was conducted according to the Declaration of Helsinki principles.
REFERENCES
1. McDonald JB, Dueck AC, Gray RJ, Wasif N, Swanson DL, Sekulic A, Malignant melanoma in the elderly: different regional disease and poorer prognosisJ Cancer 2011; 2: 538-43.
2. Lasithiotakis K, Leiter U, Meier F, Eigentler T, Metzler G, Moehrle M, Age and gender are significant independent predictors of survival in primary cutaneous melanomaCancer 2008; 112: 1795-804.
3. Adamkov M, Lauko Ľ, Rajčáni J, Bálentová S, Rybárová S, Misˇtuna D, Expression of antiapoptotic protein survivin in malignant melanomaBiologia 2009; 64: 840-4.
4. Crowson AN, Magro CM, Mihm MC, jrPrognosticators of melanoma, the melanoma report, and the sentinel lymph nodeMod Pathol 2006; 19: 71-87.
5. Chao C, Martin RC, 2ndRoss MI, Reintgen DS, Edwards MJ, Noyes RD, Correlation between prognostic factors and increasing age in melanomaAnn Surg Oncol 2004; 11: 259-64.
6. Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging systemJ Clin Oncol 2001; 19: 3622-34.
7. Testori A, Soteldo J, Sances D, Mazzarol G, Trifirò G, Zonta M, Cutaneous melanoma in the elderlyMelanoma Res 2009; 19: 125-34.
8. Austin PF, Cruse W, Lyman G, Schroer K, Glass F, Reintgen DS, Age as a prognostic factor in the malignant melanoma populationAnn Surg Oncol 1994; 1: 487-94.
9. Caraco C, Marone U, Botti G, Celentano E, Lastoria S, Mozzillo N, Age as predictor in patients with cutaneous melanoma submitted to sentinel lymph node biopsyEur J Surg Oncol 2006; 32: 970-3.
10. Hanrahan PF, Hersey P, D’Este CA, Factors involved in presentation of older people with thick melanomaMed J Aust 1998; 169: 410-4.
11. Loggie B, Ronan SG, Bean J, Das Gupta TK, Invasive cutaneous melanoma in elderly patientsArch Dermatol 1991; 127: 1188-93.
12. Swetter SM, Geller AC, Kirkwood JM, Melanoma in the older personOncology (Williston Park) 2004; 18: 1187-96.discussion 1196-7
13. Sobin LH, Gospodarowicz MK, Wittekind Ch, TNM Classification of Malignant Tumours2009; 7th Edition. Wiley-Blackwell; 336-ISBN: 978-1-4443-3241-4
14. Mervic L, Time course and pattern of metastasis of cutaneous melanoma differ between men and womenPLoS One 2012; 7: e32955.
15. Purdue MP, From L, Armstrong BK, Kricker A, Gallagher RP, McLaughlin JR, Etiologic and other factors predicting nevus-associated cutaneous malignant melanomaCancer Epidemiol Biomarkers Prev 2005; 14: 2015-22.
16. Bevona C, Goggins W, Quinn T, Fullerton J, Tsao H, Cutaneous melanomas associated with neviArch Dermatol 2003; 139: 1620-4.
17. Chamberlain AJ, Fritschi L, Giles GG, Dowling JP, Kelly JW, Nodular type and older age as the most significant associations of thick melanoma in Victoria, AustraliaArch Dermatol 2002; 138: 609-14.
18. Tejera-Vaquerizo A, Barrera-Vigo MV, Lopez-Navarro N, Herrera-Ceballos E, Growth rate as a prognostic factor in localized invasive cutaneous melanomaJ Eur Acad Dermatol Venereol 2010; 24: 147-54.
19. Balch CM, Thompson JF, Gershenwald JE, Soong SJ, Ding S, McMasters KM, Age as a predictor of sentinel node metastasis among patients with localized melanoma: an inverse correlation of melanoma mortality and incidence of sentinel node metastasis among young and old patientsAnn Surg Oncol 2014; 21: 1075-81.
20. Jukic DM, Rao UN, Kelly L, Skaf JS, Drogowski LM, Kirkwood JM, Microrna profiling analysis of differences between the melanoma of young adults and older adultsJ Transl Med 2010; 8: 27.
21. Busam KJ, Murali R, Pulitzer M, McCarthy SW, Thompson JF, Shaw HM, Atypical spitzoid melanocytic tumors with positive sentinel lymph nodes in children and teenagers, and comparison with histologically unambiguous and lethal melanomasAm J Surg Pathol 2009; 33: 1386-95.
22. Berk DR, LaBuz E, Dadras SS, Johnson DL, Swetter SM, Melanoma and melanocytic tumors of uncertain malignant potential in children, adolescents and young adults – the Stanford experience 1995–2008Pediatr Dermatol 2010; 27: 244-54.
Notes
Source of Support: Nil
Conflict of Interest: None declared.
Comments are closed.