Our Dermatol Online. 2014; 5(4): 391-394
DOI:. 10.7241/ourd.20144.98
Date of submission: 29.07.2014 / acceptance: 10.09.2014
Conflicts of interest: None
A COMPLEX IMMUNE RESPONSE IN HALO NEVI CORRELATES WITH IMMUNE REACTIVITY ON INFILTRATED MELANOCYTES, ADJACENT HAIR FOLLICLES AND BLOOD VESSELS
Ana Maria Abreu Velez1, Vickie M. Brown2, Michael S. Howard1
1Georgia Dermatopathology Associates, Atlanta, Georgia, USA
2Family Dermatology, Milledgeville, Georgia, USA
Corresponding author: Ana Maria Abreu Velez, M.D., Ph.D., e-mail: abreuvelez@yahoo.com
How to cite this article: Abreu Velez AM, Brown VM, Howard MS. A complex immune response in halo nevi correlates with immune reactivity on infiltrated melanocytes, adjacent hair follicles and blood vessels. Our Dermatol Online. 2014; 5(4): 391-394.
Abstract
Introduction: A clinical “halo nevus” is a benign melanocytic-neoplasm, often exhibiting spontaneous involution. A characteristic clinical feature is depigmentation of the surrounding skin, and a centripetal progression of the tumor regression phenomenon. Case Report: An 18 year old male consulted the dermatologist for changes in color of an asymptomatic mole.
Materials and Methods: A clinical evaluation was performed, and skin biopsies were obtained for hematoxylin and eosin (H&E) review, and for immunohistochemical (IHC) studies including CD3, CD4, CD8, CD20, CD68, CD99, myeloid/histiocyte antigen, S-100, PNL2 and SOX-10.
Results: A neoplastic process was identified on H&E examination, located along the dermal/epidermal junction and within the dermis. The neoplasm was composed of nests, cords and strands of benign melanocytes, with infiltrating lymphocytes. IHC staining demonstrated a strong pattern of positivity with all of the IHC antibodies within, infiltrating and surrounding the primary neoplastic process. In addition, evidence of the primary tumor immune response was noted around surrounding blood vessels and hair follicles, and on adjacent epidermal melanocytes.
Conclusions: In the present study, we demonstrate by histopathologic and immunologic evidence that lymphocytes are primarily responsible for halo nevus tumor regression. Moreover, the immune response involves not only CD8 positive T lymphocytes, but a larger spectrum of B and T lineage lymphocytes. Thus, the immunologic foundations of halo nevus regression are likely of greater complexity than previously determined..
Key words: Halo nevus; CD4; CD8; CD20; CD68; myeloid/histiocyte antigen
Abbreviations and acronyms: Bullous pemphigoid (BP), immunohistochemistry (IHC), direct and indirect immunofluorescence (DIF, IIF), hematoxylin and eosin (H&E).
Introduction
A clinical halo nevus, also known as leukoderma acquisitum centrifugum, perinevoid vitiligo and Sutton’s nevus is a melanocytic nevus surrounded by a depigmented ring or “halo” [1-5]. The formation of the halo is thought to occur when certain CD8 positive T lymphocytes appear in a lichenoid band below the nevus and destroy the melanocytes. The precise triggers of the CD8 positive lymphocytic attack are undetermined [1-5]. Halo nevi are considered primarily of cosmetic significance; thus, often no treatment is required [1-5]. Although halo nevi seem to be harmless, it is important to monitor these lesions regularly to detect changes in their appearances [1-5]. The current medical literature suggests that if there is a change in appearance, or the halo nevus becomes painful, itchy, or infected, a physician should be consulted [1-5].
Case Report
A 18-year-old male visited the dermatologist, presenting with a 2-week history of a whitening nevus on the back without any other symptoms. Skin biopsies for hematoxylin and eosin (H & E) examination and for immunohistochemical (IHC) studies were performed.
Material and Methods
Our IHC staining was read as positive or negative, in the presence of both negative and positive controls for each marker tested. The readings were performed by an immunodermatologist and were based on the stain positivity of the tumor on 200 and 400X magnification, as previously described [6-8]. The following antibodies were tested: CD3, CD4, CD8, CD20, CD68, CD99, S100, PNL2 (melanocyte specific antigen), myeloid/histiocyte antigen and SOX-10 (a neural crest transcription factor). Staining was performed as previously described [6-8].
Results
Microscopic Description
Review of the hematoxylin and eosin tissue sections demonstrated a melanocytic neoplastic process located along the dermal/epidermal junction and within the dermis. The neoplasm was composed of nests, cords and strands of benign melanocytes. Dermal melanocytic mitotic figures are rare. A band-like, lichenoid infiltrate of lymphocytes and histiocytes was present immediately subjacent to the lesion, and also infiltrating some of the lesional melanocytes (Fig. 1). No dysplastic histologic features are appreciated. The lesion appeared free of the specimen margins in the sections examined.
IHC staining
A strong infiltrate of CD3 and CD8 positive cells was present in a lichenoid pattern subjacent to and infiltrating the lesional melanocytes; in addition, these markers were noted around selected adjacent dermal blood vessels and around perilesional junctional melanocytes. The CD3 and CD8 positive cell populations had similar patterns and intensity of positivity (Figs 2, 3). CD4 positive cells were also observed, in a similar pattern as the CD3 and CD8 positive cells, but with decreased numbers of positive cells per square millimeter of tissue. CD20 positive cells were appreciated, in a similar pattern as the above patterns; however, these cells were noted in greatly decreased numbers relative to CD3, CD4 and CD8. CD68 positive cells were noted infiltrating lesional melanocytes. S100 positive staining was noted on melanocytes, and on Langerhans antigen presenting cells. PNL2 and SOX-10 positive staining was noted on melanocytes. We also noted CD99 positive cells in similar locations as the T cells. Specifically, CD99 staining was noted around the primary melanocytic nevus and on hair follicular units, both in the isthmus and around hair follicle bulb melanocyte. Myeloid/histoid antigen staining was positive around the primary nevus, and on adjacent hair follicle isthmus areas.
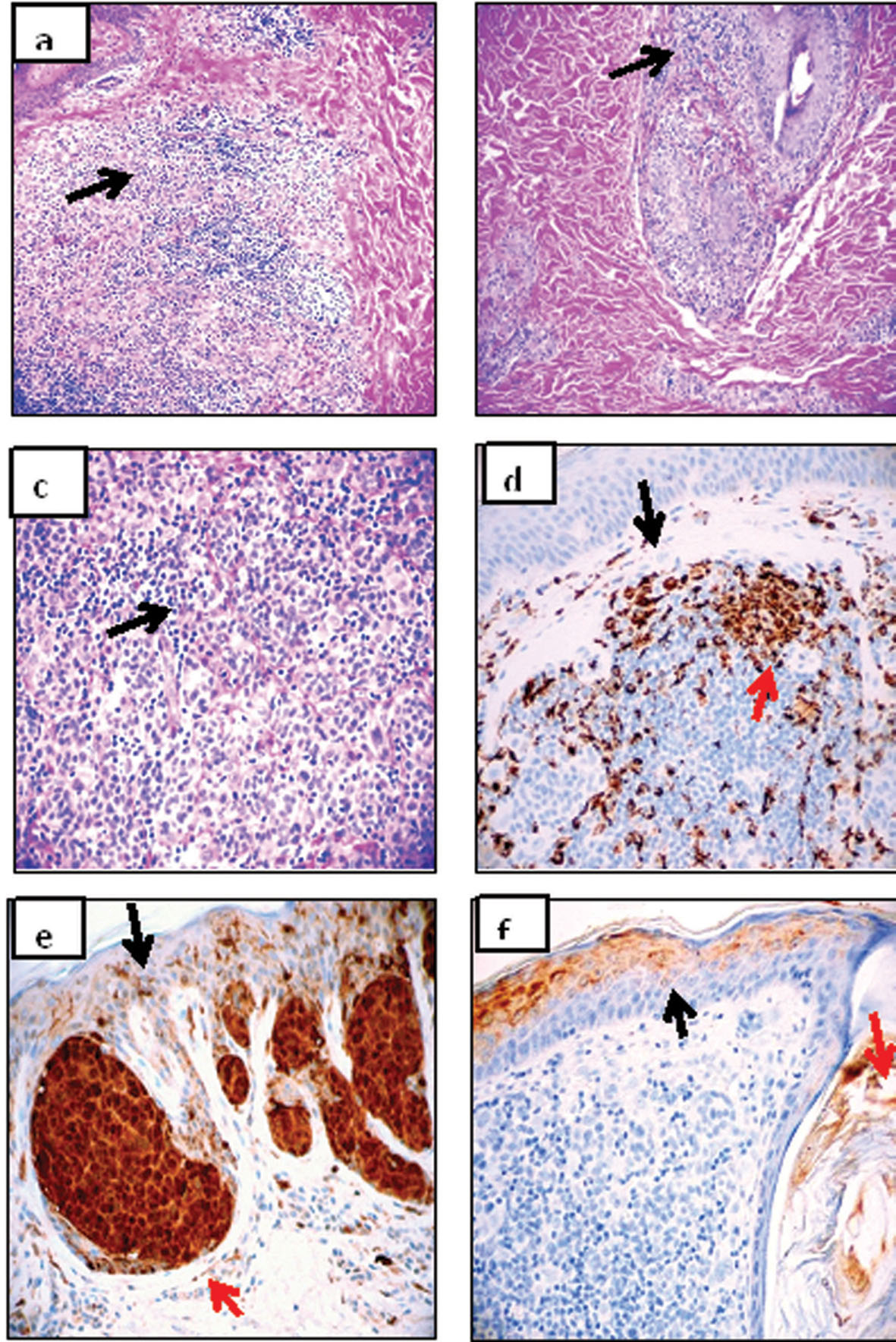 Figure 1. a, H&E stain showing a mixed population of melanocytes, lymphocytes and histiocytes in the halo nevus (black arrow) (40x). b. A detail showing that the lymphocytes and histiocytes approach an adjacent hair follicular unit (black arrow)(200x). c. Similar to a, but at higher magnification (black
arrow)(400x). d. IHC stain showing CD68 positive cells both in the dermis below the halo nevus (black arrow), and within the halo (red arrow)(brown staining, 200x). e. IHC, demonstrating positive S-100 staining in the halo nevus melanocytes (red arrow) and above the nevus, scattered in the epidermis(black arrow)(brown staining) (200x). f. IHC stain, showing positivity with myeloid/histoid antigen antibody above the halo nevus in the epidermis (diffuse brown staining; black arrow) and in the hair isthmus (red arrow)(200x). |
 Figure 2. a, Positive IHC staining with PNL2 (black arrow; brown staining). The red arrow highlights staining at the basement membrane zone (100x). b. Positive IHC staining with CD68(black arrow; brown staining)(200x). c. Positive IHC staining with CD3 (black arrow; brown staining)(200x). d. Positive IHC staining with CD4 (black arrow; brown staining) (200x). e. Positive IHC staining with CD99 (black arrow; brown staining) (200x). f. Positive IHC staining with CD20 (black arrow; brown staining)(200x).
|
 Figure 3. a. Positive IHC staining with SOX-10 on halo nevus melanocytes (brown staining, black arrow) (40x). b. Same SOX- 10 IHC staining at the lower edge of the halo nevus, showing the nevus cells(black arrow) infiltrating lymphocytes around the edge (red arrow)(200x). c. Positive IHC staining with CD20 (brown staining; black arrow). Note these B lymphocytes are not as numerous as the T lymphocytes, but present in similar areas within the infiltrate (100x). d. Positive PNL2 IHC staining of melanocytes within a hair follicle bulb, adjacent to the halo nevus (black arrow, brown staining)(400x). e. A cartoon diagram, summarizing portions of our data and detailing how T
lymphocytes and antigen presenting dendritic cells could surround melanocytes within the halo nevus and within adjacent hair follicles. |
|
Discussion
The halo nevus immune response has been previously regarded as an autoimmune process, mediated primarily by CD8 positive T lymphocytes. Specifically, the CD8 positive T cells are thought to mediate a progressive destruction of nevus cells. Halo nevi may be associated with other autoimmune disorders such as vitiligo, Hashimoto’s thyroiditis, alopecia areata, celiac disease and atopic dermatitis. It has been previously noted that halo nevi are often detected after intense sun exposure, and especially after sunburns [3,9]. Minimal information is present win the medical literature regarding specifics of the immune response in halo nevi. On review, we found less than 5 citations specifically confirming the CD8 lymphocytic immune response. One report cited the presence of IgM in lesional skin [10]. Although no direct demonstration of melanocyte destruction has been observed by specific immune effector cells found within the halo, the 1) abundance of antigen-presenting cells in the regressing nevus and 2) presence of T lymphocytes at the site of depigmentation suggest that these cells also participate in the halo immune response. In our case, we were able to further confirm the presence of CD68 positive and myeloid histoid antigen positive cells in and around the halo nevus. In conjunction with these T lymphocyte, Langerhans cell and non- Langerhans histiocyte populations, substantial evidence points to the involvement of CD8 positive T lymphocytes as end line effectors in the destruction of halo nevus melanocytes [1-4]. The specific triggers of the autoimmune breakdown of tolerance that triggers the migration and the presumed activation of these CD8 positive lymphocytes in the nevus (in the apparent absence of disease) are unknown. Selected authors have performed electron microscopic studies, and reported findings of lymphocyte, monocyte, and plasma cell infiltration of halo nevi followed by vacuolar cytolysis. These findings support the concept of a sustained autoimmune reaction in regressing halo nevi [5]. We observed multiple positive antigen presenting cell markers; these markers were present not only around and between the melanocytes of the halo nevus, but also in proximity to adjacent hair follicular units. These immune response details are, to our knowledge, previously undocumented. We also noted that CD99 (also known as MIC2 or single-chain type- 1 glycoprotein) marked positively within the halo nevus cells. CD99 (a glycosylated transmembrane protein expressed on all leukocytes and most strongly on thymocytes) is believed to augment both T cell adhesion and apoptosis of T cells. Because we present a single case, we suggest that the immune response in halo nevi is likely not as simple as a solitary, cytotoxic effect of CD8 positive T lymphocytes on melanocytes. Moreover, we suggest that the other positive markers(and correlating cells) noted in our study do not represent simple epiphenomena. We suggest that these additional cells may not be responsible for the direct destruction of the nevus melanocytes. Further studies are required to confirm and clarify the precise roles of these cells in the overall halo nevus immune response.
REFERENCES
1.Nazzaro G, Rovaris M. The men or women behind nevi: Richard Sutton. JAMA Dermatol. 2014;150:302.
2.Steffen C, Thomas D. The man behind the eponyms: Richard L. Sutton: periadenitis mucosa necrotica recurrens (Sutton’s ulcer) and leukoderma acquisitum centrifugum-Sutton’s (halo) nevus. Am J Dermatopathol. 2003;25:349-54.
3. Zeff RA, Freitag A, Grin CM, Grant-Kels JM. The immune response in halo nevi. J Am Acad Dermatol. 1997;37:620-24.
4. Pustisek N, Sikanić-Dugić N, Hirsl-Hećej V, Domljan ML. „Halo nevi” and UV radiation. Coll Antropol. 2010;34 Suppl 2:295-7.
5. Jacobs JB, Edelstein LM, Snyder LM, Fortier N. Ultrastructural evidence for destruction in the halo nevus. Cancer Res. 1975;35:352-7.
6. Abreu Velez AM, Howard MS, Pereyo NY, Delman KA, Mihm MC, Rizzo M. Lymphohistiocytic CD45/CD8 immune response in a case of malignant melanoma, with possible cannibalism. North Am J Med Sci. 2012;4:507-9.
7. Abreu Velez AM, Googe PB, Howard MS. Immunohistochemistry versus immunofluoresence in the diagnosis of autoimmune blistering diseases. Our Dermatol Online. 2013;4(Suppl.3):627-30.
8. Abreu Velez AM, Howard MS. Immune reactivity in psoriatic Munro-Saboureau microabscesses, stratum corneum and blood vessels. North Am J Med Sci. 2012;4:257-65.
9. Becker MD, Marcks KM, Trevaskis AE, Heffernan AG, Puchner G. Halo nevus of Sutton. Plast Reconstr Surg. 1966;37:413-5.
10. Tokura Y, Yamanaka K, Wakita H, Kurokawa S, Horiguchi D, Usui A, et al. Halo congenital nevus undergoing spontaneous regression. Involvement of T-cell immunity in involution and presence of circulating anti-nevus cell IgM antibodies. Arch Dermatol. 1994;130:1036-41.
11. Rocchi A, Manara MC, Sciandra M, Zambelli D, Nardi F, Nicoletti G, et al. CD99 inhibits neural differentiation of human Ewing sarcoma cells and thereby contributes to oncogenesis. J Clin Invest. 2010;3:668–80.
Comments are closed.