Our Dermatol Online. 2013; 4(2): 221-223
DOI:. 10.7241/ourd.20132.54
Date of submission: 26.02.2013 / acceptance: 20.03.2013
Conflicts of interest: None
SYRINGOCYSTADENOCARCINOMA PAPILLIFERUM: A CASE REPORT OF A RARE SKIN ADNEXAL TUMOUR
Chidambharam Choccalingam1, Premila Samuel1, Deepak Subramaniam2, Faizal Hammed2,
Purushothaman V2, Rajiv Joshi3
1Department of Pathology, SRL Ltd, Chennai, India
2Department of Surgery and Plastic Surgery, Fortis Malar, Chennai, India
3Department of Dermatology, SRL Ltd, Mumbai, India
Corresponding author: Dr Chidambharam Choccalingam e-mail: Chidambharam@gmail.com
How to cite an article: Choccalingam C, Samuel P, Subramaniam D, Hammed F, Purushothaman V, Joshi V. Syringocystadenocarcinoma Papilliferum: A case report of a rare skin adnexal tumour. Our Dermatol Online. 2013; 4(2): 221-223.
Abstract
Syringocystadenocarcinoma papilliferum (SCACP), a rare skin adnexal carcinoma with apocrine differentiation is the malignant counterpart of syringocystadenoma papilliferum (SCAAP). It usually occurs in the head and neck region of elderly individuals. We describe a 46 year old south Indian female with a lesion in the scalp. Morphologically the tumour had the characteristic features of SCAAP along with frank invasion into deep dermis and malignant cytologic features. Immunohistochemically, the tumour cells stained strongly with cytokeratin (CK) 7, carcinoembryonic antigen (CEA), gross cystic disease fluid protein-15 (GCDFP-15), We made a diagnosis of SCACP in the patient, and a wide excision with skin grafting was performed on the patient.
Key words: syringocystadenocarcinoma papilliferum; adnexal neoplasm; apocrine differentiation
Introduction
Syringocystadenocarcinoma papilliferum (SCACP) is a rare skin adnexal carcinoma, considered to be a malignant counterpart of syringocystadenoma papilliferum (SCAAP) by the World Health Organization [1,2]. Adnexal tumours are skin tumours whose differentiation is towards one or more of the cutaneous adnexal structures (apocrine, follicular, and sebaceous) of the skin. SCACP is a malignant adnexal neoplasm that shows predominantly apocrine differentiation [3]. It is an extremely rare cutaneous neoplasm with only 13 reported cases, to our knowledge until 2012, since it was first reported in 1980 [2,4]. Most of the SCACP’s is preceded by long standing SCAAP. The malignant tumour has a predilection for the head and neck region and commonly affects older people in the 5th to 7th decade [3,4]. To our knowledge, only one case of SCACP has been reported from India [4]. Herein we report a case of SCACP occurring on the right scalp of 46 year old South Indian female patient.
Case Report
A 48 year old south Indian woman presented with an ulcerative growth over the scalp for duration of 1 year (Fig. 1). Since the clinical diagnosis was in favour of squamous cell carcinoma, a needle core biopsy was performed. Histopathologic examination revealed cystic invaginations and papillomatous downgrowths extending up to the deep dermis. The cystic invaginations and papillomatous downgrowths were lined by luminal high columnar cells, with few showing decapitation secretion and outer cuboidal cells (Fig. 2a). The cells show moderate to severe atypia with increased mitotic activity (Fig. 2b). There was a dense infiltration of abundant plasma cells and lymphocytes in the stroma of the tumour. In few loci, frank invasion of the stroma by the tumour was seen. Based on these histopathological findings, the diagnosis of SCACP was made. Wide excisional surgery with split skin graft was performed to remove the tumour. There were no signs of lymph node involvement ultrasonographically and clinically. The patient had no clinical or radiographic evidence of any primary tumour elsewhere. Gross pathology of the whole specimen revealed a skin ellipse with a large asymmetric ulcerative growth measuring 6 * 5 * 2 cms. The growth was poorly circumscribed and seen extending up to the dermis. The histopathological examination of the whole growth revealed the same, with stromal invasion being prominent. Immunohistochemically, the tumour cells stained strongly with cytokeratin (CK) 7, carcinoembryonic antigen (CEA), gross cystic disease fluid protein-15 (GCDFP-15), which further provided support to the apocrine nature of the neoplasm (Fig. 3a, b, c). The margins were found to be free of tumour.
 Figure 1. Clinical photograph of the ulceroproliferative growth over the right scalp
|
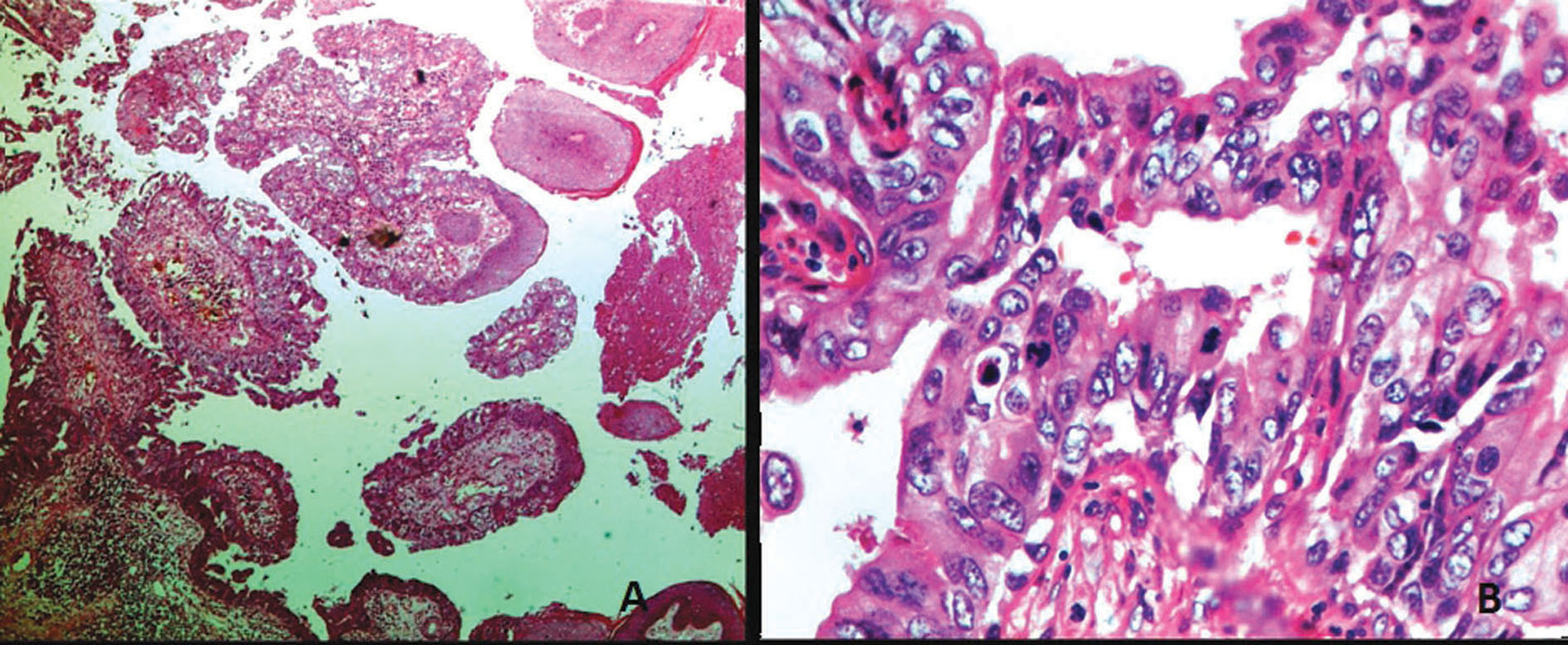 Figure 2. Biopsy from lesion revealed (A) large cystic invaginations and papillomatous downgrowths lined by inner columnar and outer cuboidal cells (Haematoxylin and Eosin X 10); (B) tumour cells with moderate to severe atypia with increased mitotic activity (Haematoxylin and Eosin X 40)
|
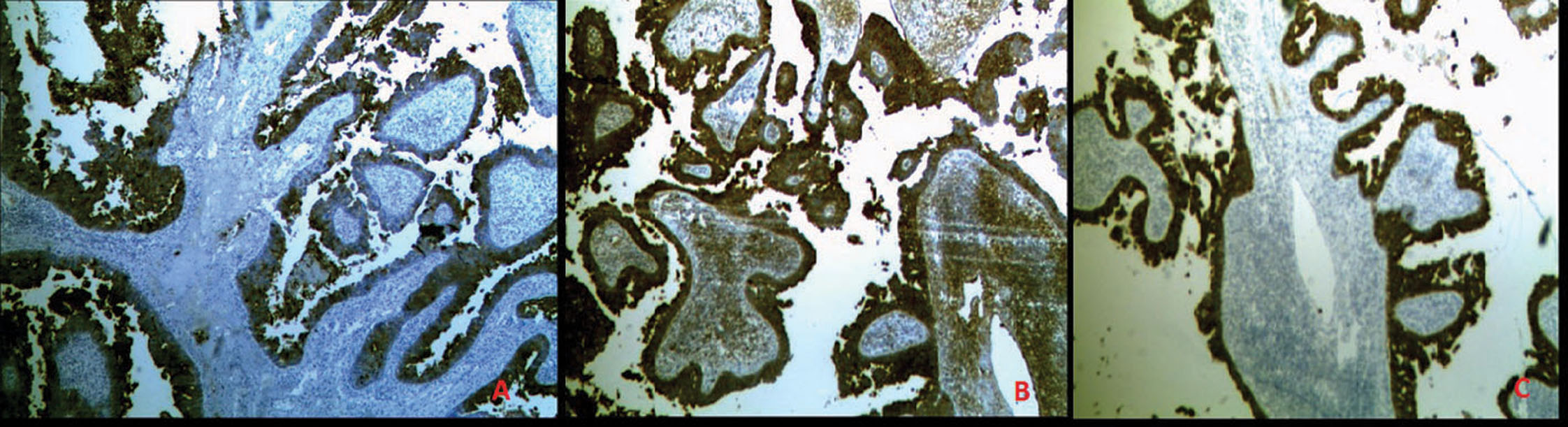 Figure 3. Immunohistochemical characterization of tumour shows (A) GCFDP-15 positive staining of tumour cells; (B) CEA positive staining of tumour cells; (C) CK7 positive staining of tumour cells
|
Discussion
SCACP is one of the cutaneous adnexal neoplasm, and is a rare neoplasm showing apocrine differentiation [3]. The apocrine histiogenesis is supported by decapitation of the luminal surface of tall columnar cells, continuity of the tumour to pilo-sebaceous units and presence of apocrine glands in the underlying tissue [3]. It is considered to be a malignant counterpart of the SCAAP. Clinically, the long standing lesion suddenly begins to enlarge in size with bleeding, crusting and ulceration. It is most commonly seen in the head and neck region of elderly individual with no gender predilection [1,3]. Morphologically, SCACP resembles SCAAP with cystic papillomatous invaginations connected to the skin surface by funnel shaped structures lined by infundibular epithelium. The upper part of the cystic invaginations are lined by keratinizing squamous epithelium while the lower part and papillary projections are lined by inner columnar cells with decapitation and outer cuboidal cells. This epithelial transition from keratinizing squamous epithelium to glandular lining recapitulates the physiologic relationship of the apocrine gland to the hair follicle. In healthy skin, apocrine gland arises at the follicular infundibulum, characterized by a gradual transition from stratified squamous epithelium at the skin surface to the bi-layered ductal structures in the dermis [1,3,6]. Stroma of the tumour contains a dense inflammatory infiltrate of plasma cells and lymphocytes mirroring the attraction of plasma cells by glands of the normal secretory immune system [5]. SCACP differs from SCAAP and SCAAP IN SITU by infiltration of tumour cells into deep dermis or subcutaneous fat. SCACP and SCAAP IN SITU differ from SCAAP by cytological features of tumour cells characterized by higher nuclear cytoplasmic ratio, nuclear irregularity, coarse chromatin and increased mitotic activity [1,3]. Though there is no definitive immunohistochemical profile for a SCACP, the strong expression of CK7, CEA and GCFDP-15 supports the apocrine differentiation of the neoplasm [1]. It is well established that SCAAP shows positivity to CEA, CK7 and epithelial membrane antigen (EMA) in the luminal cells. However only 2 out of the 4 SCACP’s on which GCFDP-15 expression was evaluated, showed positive expression. Though there is not many published literature on the immunohistochemical characterization of SCACP, it is assumed that SCACP mirrors the immunohistochemical profile of SCAAP [7]. Our case was identified as SCACP since it showed the characteristic histology along with infiltration into deep dermis and presence of malignant cells. Also, the tumour strongly expressed apocrine differentiation markers CK7, CEA and GCFDP-15. Though a rare neoplasm, the entity should be recognized correctly as it may affect patient treatment and prognosis. Similar to other rare adnexal tumours, the literature reveals a good prognosis for SCACP patients treated only by surgical excision [8-10].
Acknowledgement
Department of Histopathology, SRL Mumbai for performing the immunohistochemical characterization of the tumour.
REFERENCES
1. Leeborg N, Thompson M, Rossmiller S, Gross N, White C, Gatter K: Diagnostic Pitfalls in Syringocystadenocarcinoma Papilliferum: Case Report and Review of the Literature. Archives of Pathology & Laboratory Medicine. 2010;134:1205-9. 2.Numata M, Hosoe S, Itoh N, Munakata Y, Hayashi S, Maruyama Y: Syringadenocarcinoma papilliferum. J Cutan Pathol. 1985;12:3-7. 3. Park SH, Shin YM, Shin DH, Choi JS, Kim KH: Syringocystadenocarcinoma Papilliferum: A Case Report. J Korean Med Sci. 2007;22:762-5. 4. Abrari A, Mukherjee U: Syringocystadenocarcinoma papilliferum at unusual site: inherent lesional histologic polymorphism is the pathognomon. BMJ Case Reports. 2011;11:4254. 5. LeBoit PE, Burg G, Weedon D, Sarasin A, editors. Pathology and Genetics of Skin Tumours. Lyon: IARC; 2006. 6. Lindboe C, Brekke H, Schonhardt I, Houge U: Syringocystadenocarcinoma Papilliferum In Situ: Case Report with Immunohistochemical Observations. Scholarly Research Exchange. 2009;2009:1-4. 7. Ishida-Yamamoto A, Sato K, Wada T, Takahashi H, Iizuka H: Syringocystadenocarcinoma papilliferum: Case report and immunohistochemical comparison with its benign counterpart. J Am Acad Dermatol. 2001;45:755-9. 8. Dissanayake RVP, Salm R. Sweat-gland carcinomas: prognosis related to histological type. Histopathology. 1980;4:445-66. 9. Santa Cruz DJ: Sweat gland carcinomas: a comprehensive review. Semin Diagn Pathol. 1987;4:38-74. 10. Mlika M, Chelly B, Boudaya S, Ayadi-kaddour A, Kilani T, El Mezni F: Poroid hidradenoma: a case report. Our Dermatol Online. 2012;3:43-5.
Comments are closed.