Our Dermatol Online. 2011; 2(4): 211-215
Date of submission: 14.02.2011 / acceptance: 23.07.2011
Conflicts of interest: None
LAT, EGFR -pY197, PCNL2, CDX2, HLA-DPDQDR, BROMODEOXYURIDINE, JAM-A, AND EZRIN IMMUNOREACTANTS IN A RUBBED SPONGIOTIC DERMATITIS
Ana Maria Abreu Velez1, A. Deo Klein2, Michael S. Howard1
1Georgia Dermatopathology Associates, Atlanta, Georgia, USA.
2Statesboro Dermatology, Statesboro, Georgia, USA
Corresponding author: Ana Maria Abreu-Velez, MD PhD e-mail: 14.02.2011 / acceptance: 23.07.2011
How to cite an article: Abreu Velez AM, Klein AD, Howard MS. LAT, EGFR -pY197, PCNL2, CDX2, HLA-DPDQDR, bromodeoxyuridine, JAM-A, and ezrin immunoreactants in a rubbed spongiotic dermatitis. Our Dermatol Online 2011; 2(4): 211-215.
Abstract
Background: Acute and subacute spongiotic dermatitides are among the most commonly diagnosed types of dermatitis. Many patients rub their lesions, with the lesions becoming clinically thickened. The precise immunologic mechanisms within the thickening process are not well defined. Case report: An 85 year old male presented with the sudden clinical appearance of erythematous patches and small blisters on the back of his legs, with pruritis. Methods: Skin biopsies, one from a rubbed lesion and one from a non-rubbed lesion were submitted for hematoxylin and eosin (H&E), immunohistochemistry (IHC), and for direct immunofluorescence (DIF) analysis. Results: The H&E staining demonstrated classic features of a spongiotic dermatitis, but in the rubbed areas psoriasiform hyperplasia was also seen. The psoriasiform areas demonstrated positive, focal IHC staining with bromodeoxyuridine, LAT, EGFR-pY197, PCNL2, CDX2, and HLA-DPDQDR antibodies. DIF staining revealed positive staining of JAM-A and ezrin in the non-rubbed specimens in both the spongiotic epidermis and in the adjacent vessels; normal expression of these markers was appreciated in the rubbed biopsy. Conclusions: The immune response seems to be complex when a spongiotic dermatitis is converted from a non-rubbed to a rubbed lesion with histologic features of psoriasiform hyperplasia.
Key words: spongiotic dermatitis; LAT; EGFR-pY197; PCNL2; CDX2; HLA-DPDQDR; bromodeoxyuridine; JAM-A; ezrin; sweat and sebaceous glands
Introduction
The spongiotic dermatoses, including eczema and its clinicopathologic variants are capable of multiple clinical presentations and are pathologically defined by the presence of epidermal edema that may infrequently form blisters. The immunologic mechanisms which underlie the pathogenesis of these disorders have only recently begun to be elucidated [1,2]. Spongiotic dermatitis may occur as a result of an allergic reaction. Such allergic reactions include an allergic contact reaction, an allergic reaction to food or a medication, or other allergic reactions. Spongiotic dermatitis is occasionally categorized clinically as eczema. Spongiotic dermatitis may be categorized histologically acute or subacute[1,2]. The main difference between histologic acute and subacute spongiotic dermatitis concerns the size of the vesicles developed by the intercellular edema. Specifically, an acute spongiotic dermatitis has larger vesicles compared with a subacute spongiotic dermatitis [1,2]. Similar to many clinical types of dermatitis, the presenting symptom is often a pruritis. After a variable clinical period, the rash appears [1,2]. The rash is usually initially erythematous, and often turns brown after repetitive rubbing or scratching of the affected areas [1,2]. Histologically, rubbed acute and subacute spongiotic dermatitides commonly develop psoriasiform hyperplasia, with additional hyperkeratosis [1,2]. Few studies have undertaken a simultaneous, comparative examination of non-rubbed acute and lichenified, rubbed lesions in the same patient. Thus, we obtained skin biopsies for hematoxylin and eosin (H&E), direct immunofluoresence (DIF) and immunohistochemistry (IHC) analyses simultaneously from both types of lesions. Our IHC staining was performed as previously described. We conducted IHC staining for LAT, survivin, epidermal growth factor receptor pY197 (EGFR-pY197), the proliferating cell nuclear antigen (PCNA), caudal type homeobox transcription factor 2 (CDX2), complement C5b-9 (MAC), junctional adhesion molecule (JAM-A), ezrin and HLA-DPDQDR, as previously described [3,6]. Our monoclonal antibodies were obtained from Dako North America (Carpinteria, California, USA), with the exception of JAM-A and ezrin (Invitrogen, Carlsbad, California, USA).
Case Report
A 85 year old male presented with rapidly appearing, inflammatory patches and plaques on the backs of his legs. Clinical examination demonstrated large, hyperkeratotic plaques, as well as separate patch areas with edema and evidence of surrounding inflammation. Lesional skin biopsies were taken from both types of lesions for hematoxylin and eosin (H&E) analysis, for immunohistochemistry (IHC) and for direct immunofluorescence (DIF) comparative studies.
Results
Microscopic description:
Examination of the H&E tissue sections in the non-rubbed biopsy demonstrated diffuse, moderate epidermal spongiosis. The dermis displayed a mild, superficial, perivascular infiltrate of lymphocytes, histiocytes and eosinophils; neutrophils were rare. No vasculitis was present; focal dermal edema was noted around the eccrine and sebaceous glands. The rubbed biopsy demonstrated hyperparakeratosis with overall psoriasiform hyperplasia; focal areas of mild spongiosis were noted, with one small subcorneal blister. Dermal edema surrounding the eccrine and sebaceous glands was also noted. DIF of the non-rubbed biopsy displayed the following results: IgG (-); IgG3 (-); IgG4 (-); IgA (-); IgM (-); IgD (-); IgE (-); Complement/C1q (-);Complement/C3 (-); collagen IV (CIV) (++, normal distribution); JAM-A and Ezrin (+, some accentuation near the basement membrane zone (BMZ) and in the spongiotic epidermis). Albumin (-), fibrinogen (++, around upper dermal blood vessels and eccrine glands). The DIF of the rubbed biopsy was mostly negative (Fig. 1,2). The IHC studies in the rubbed biopsy were patchy positive in areas where the spongiosis seemed to be transitioning into psoriasiform hyperplasia. Some alterations were also seen around dermal eccrine and sebaceous glands, and around dermal blood vessels (Fig. 1,2). In both the non-rubbed and rubbed biopsies, the IHC stains for LAT, EGFR-pY197, bromodeoxyuridine, MAC, PCNA, CDX2, and HLA-DPDQDR were focally positive, primarily in areas of the previously described hyperparakeratosis. .
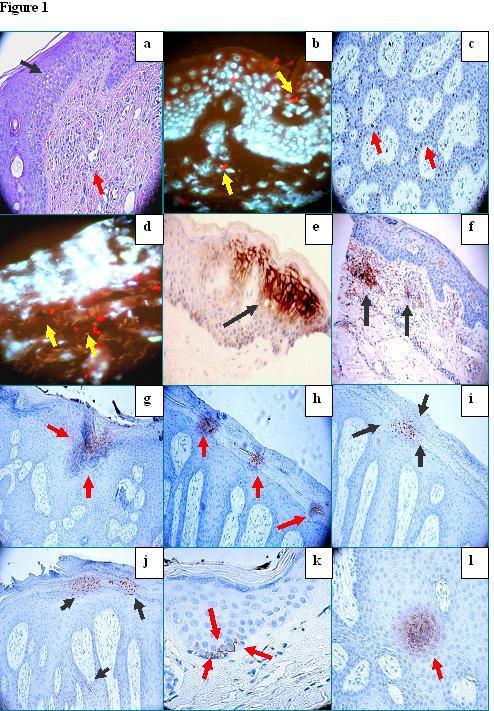 Figure 1. a. H&E demonstratingm focally marked epidermal spongiosis(black arrow) with diffuse edema in the papillary dermis and inflammatory infiltrates around the upper dermal blood vessels (red arrow). b. DIF demonstrating focal areas staining positive for Texas red conjugated JAM-A (red staining; yellow arrows) in the epidermis as well as in dermal perivascular areas. Keratinocyte and other cell nuclei were counterstained with 4′,6′ diamino-2-phenylindole (Dapi) (bluewhite staining). c. IHC demonstrating positive staining with PCNL2 antibody in several cells along the basement membrane zone of the epidermis (brown staining, red arrows).d. DIF demonstrating positive staining with Texas red conjugated ezrin (red stain) in several perivascular areas of the upper dermis (red staining; yellow arrows). Nuclei were again counterstained with Dapi. e. and f , IHC demonstrating positive staining with HLA-DPDQDR antibody in some of the epidermal spongiotic areas in e and in the dermal perivascular inflammatory cell infiltrate in f (brown staining, black arrows). g Positive IHC staining for LAT antibodies in a localized area in the upper epidermis where the spongiosis is prominent (brown staining; red arrows). h Positive IHC staining for CDX2 in the epidermal corneal layer (brown staining, red arrows). i. Positive IHC staining for EGFR-pY197 in focal areas of the epidermal corneal layer where rubbing has occurred (brown staining; black arrows). Notice that the epidermis is displaying having psoriasiform hyperplasia. j. Strongly positive IHC staining for PCNL2 within epidermal corneal layer with hyperkeratosis, as well as in some cells along the basement membrane zone (brown staining; black arrows). k. Positive IHC staining with bromodeoxyuridine in a cluster of cells along the epidermal basement membrane area (BMZ) (brown staining; red arrows). l. Positive IHC staining with LAT in a focal areas of the epidermal stratum spinosum (brown staining; red arrow).
|
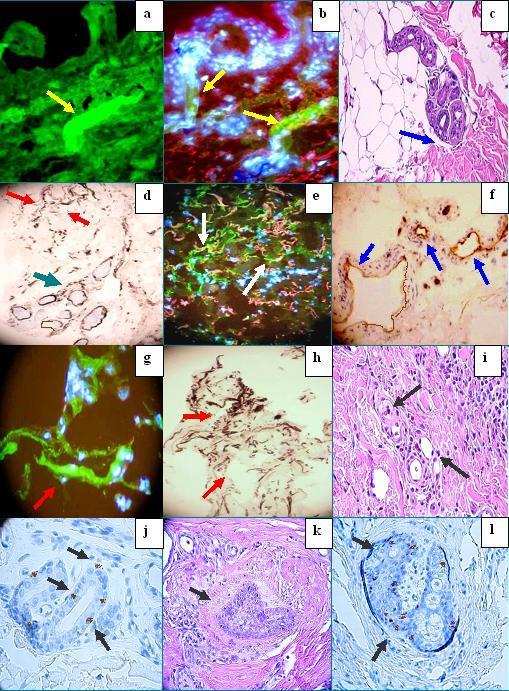 Figure 2. . a and b, Positive DIF staining with FITC conjugated fibrinogen antibodies against the dermal eccrine ductus (green staining; yellow arrows). Nuclei were counterstained with Dapi (blue-white). In b, also note positive staining for Texas red conjugated JAM-A around several upper dermal blood vessels. c. H&E demonstrating dermal edema around eccrine sweat glands (blue arrow). d. Positive IHC staining for MAC, around dermal blood vessels (red arrow), and around dermal eccrine sweat glands (green arrow). e. Positive IHC staining with anti-human FITC conjugated fibrinogen around several upper dermal vessels (green staining; white arrows). The nuclei were counterstained with Dapi. Positive staining for JAM-A is also present (pink). f. IHC positive staining with anti-human HLA-DPDQDR antibody, present around upper blood vessels (brown staining, blue arrows). g. Positive DIF staining with FITC conjugated anti-human fibrinogen around the upper dermal blood vessels (green staining, red arrow). The nuclei were counterstained with Dapi. h. Detail of positive IHC staining for MAC around upper dermal blood vessels (red arrows). i. H&E detailing some mild, chronic inflammation around upper dermal dilated blood vessels (black arrows). j and l, IHC positive staining with PCNL2 antibody in some cells of the eccrine sweat glands and sebaceous glands, respectively (brown staining, black arrows). k. H&E detail of mild inflammation and edema surrounding a portion of a sebaceous gland (black arrow).
|
Discussion
Our results indicate that the immune response is modified in acute spongiosis in rubbed areas versus nonrubbed areas. The rubbing action leads histologically to hyperkeratosis and psoriasiform hyperplasia. Based on our data, we suggest that the immune response may vary vis-à-vis acute or subacute stages in spongiotic dermatitides [8-12]. An initial immune response seems to exist to the initializating allergens, however, subsequent itching and sweating could further irritate the skin, leading to clinical individual rubbing and scratching. These actions could then in turn lead to further immune alterations, inducing further stimulation of the T cell receptor (TCR) as indicated by IHC positivity to LAT. Other pathophysiologic events seem to occur simultaneously, including cell proliferation (indicated by positive IHC staining for PCNL2 and bromodeoxyuridine) [8-12]. The rubbing of the epidermis may induce the IHC positivity seen for the epidermal growth factor receptor; further, the rubbing and/or other signals may induce inflammation around dermal blood vessels and sweat glands, as suggested here by IHC positivity for fibrinogen and other immunoreactants. More specifically, we suggest the epidermal spongiotic areas with hyperkeratotic alterations may upregulate and/or downregulate multiple cell signals that are induced by the rubbing itself [9-11]. In addition, JAM-A and ezrin (cell junction molecules) could be also be upregulated in acute spongiosis, but not in the more chronic lesions. EGPF-pY197 and PCNA seem to be expressed in the hyperkeratotic areas, suggesting creation of a cycle of rubbing and feedback increases in cell proliferation [9-11]. Potentially, the local and subtle expression of PCNA would attempt to prevent cell apoptosis in the edematous skin. To our surprise, the CDX2 antibody was also positive in same areas as EGFR-pY197 and PCNA; this molecule is strongly expressed in intestinal epithelia. However, it has been shown that the in vivo phenotype of each epithelium seems to depend on multiple factors. Specifically, the morphological and biochemical differentiation of a given epithelium may be reversibly modulated by the external environment; thus, extrinsic factors may, under certain conditions, affect the morphological and biochemical differentiation of a given epithelium. Further studies are required to properly identify these molecules, and their roles in regulating epithelial differentiation. In regard to HLA class II, it has been shown that expression of these molecules on keratinocytes has been confirmed in allergic contact dermatitis, while absent in atopic dermatitis [11]. Other authors have demonstrated keratinocyte HLA staining accompanying epidermal lymphoid infiltrates. In addition, other authors have noted evidence of focal keratinocyte damage in the midepidermis in eczema, as we have previously described [5-6]. Identifying the triggers of a spongiotic dermatitis is a challenging process of elimination. However, given our data, we recommend advising the patient to avoid rubbing clinical lesions, thus avoiding further immunologic alterations that may, in turn, autocatalyze clinical lesional development.
Conclusion
1. Gupta K: Deciphering spongiotic dermatitides. Indian J Dermatol Venereol Leprol. 2008; 74: 523-526. 2. Houck G, Saeed S, Stevens GL, Morgan MB: Eczema and the spongiotic dermatoses: a histologic and pathogenic update. Semin Cutan Med Surg. 2004; 23: 39-35. 3. Abreu-Velez AM, Howard MS, Hashimoto T, Grossniklaus HE: Human eyelid meibomian glands and tarsal muscle are recognized by autoantibodies from patients affected by a new variant of endemic pemphigus foliaceus in El-Bagre, Colombia, South America. J Am Acad Dermatol. 2010; 62: 437-447. 4. Howard MS, Yepes MM, Maldonado-Estrada JG, Villa- Robles E, Jaramillo A, Botero JH, et al: Broad histopathologic patterns of non-glabrous skin and glabrous skin from patients with a new variant of endemic pemphigus foliaceus-part 1. J Cutan Pathol. 2010; 37: 222- 230. 5. Abreu Velez AM, Howard MS, Pinto FJ Jr: Dyshidrotic eczema: relevance to the immune response in situ. North Am J Med Sci, 2009; 1: 117-120. 6. Abreu Velez AM, Howard MS, Smoller BR: Atopic dermatitis and possible polysensitization, with monkey esophagus reactivity. North Am J Med Sci. 2010; 2: 336- 340 7. Abreu-Velez AM, Klein AD, Howard MS: Junctional adhesion molecule overexpression in Kaposi varicelliform eruption skin lesions-as a possible herpes virus entry site. North Am J Med Sci 2010; 2: 433-437. 8. Abreu Velez AM, Jackson BL, Howard MS A: Deposition of immunoreactants in a cutaneous allergic drug reaction. North Am J Med Sci. 2009; 1: 180-183. 9. Dong S, Corre B, Nika K, Pellegrini S, Michel F: T Cell receptor signal initiation induced by low-grade stimulation requires the cooperation of LAT in human T cells. PLoS One. 2010; 30; e15114. 10. Lu S, Tiekso J, Hietanen S, Syrjaè Nen K, Havu VK, Syrjaè Nen S: Expression of Cell-cycle Proteins p53, p21 (WAF-1), PCNA and Ki-67 in benign, premalignant and malignant skin lesions with implicated HPV involvement. Acta Derm Venereol 1999; 79: 268-273. 11. Barker JN, MacDonald DM: Epidermal class II human lymphocyte antigen expression in atopic dermatitis: a comparison with experimental allergic contact dermatitis. J Am Acad Dermatol. 1987; 16: 1175-1179.
REFERENCES
1. Gupta K: Deciphering spongiotic dermatitides. Indian J Dermatol Venereol Leprol. 2008; 74: 523-526. 2. Houck G, Saeed S, Stevens GL, Morgan MB: Eczema and the spongiotic dermatoses: a histologic and pathogenic update. Semin Cutan Med Surg. 2004; 23: 39-35. 3. Abreu-Velez AM, Howard MS, Hashimoto T, Grossniklaus HE: Human eyelid meibomian glands and tarsal muscle are recognized by autoantibodies from patients affected by a new variant of endemic pemphigus foliaceus in El-Bagre, Colombia, South America. J Am Acad Dermatol. 2010; 62: 437-447. 4. Howard MS, Yepes MM, Maldonado-Estrada JG, VillaRobles E, Jaramillo A, Botero JH, et al: Broad histopathologic patterns of non-glabrous skin and glabrous skin from patients with a new variant of endemic pemphigus foliaceus-part 1. J Cutan Pathol. 2010; 37: 222- 230. 5. Abreu Velez AM, Howard MS, Pinto FJ Jr: Dyshidrotic eczema: relevance to the immune response in situ. North Am J Med Sci, 2009; 1: 117-120. 6. Abreu Velez AM, Howard MS, Smoller BR: Atopic dermatitis and possible polysensitization, with monkey esophagus reactivity. North Am J Med Sci. 2010; 2: 336- 340 7. Abreu-Velez AM, Klein AD, Howard MS: Junctional adhesion molecule overexpression in Kaposi varicelliform eruption skin lesions-as a possible herpes virus entry site. North Am J Med Sci 2010; 2: 433-437. 8. Abreu Velez AM, Jackson BL, Howard MS A: Deposition of immunoreactants in a cutaneous allergic drug reaction. North Am J Med Sci. 2009; 1: 180-183. 9. Dong S, Corre B, Nika K, Pellegrini S, Michel F: T Cell receptor signal initiation induced by low-grade stimulation requires the cooperation of LAT in human T cells. PLoS One. 2010; 30; e15114. 10. Lu S, Tiekso J, Hietanen S, Syrjaè Nen K, Havu VK, Syrjaè Nen S: Expression of Cell-cycle Proteins p53, p21 (WAF-1), PCNA and Ki-67 in benign, premalignant and malignant skin lesions with implicated HPV involvement. Acta Derm Venereol 1999; 79: 268-273. 11. Barker JN, MacDonald DM: Epidermal class II human lymphocyte antigen expression in atopic dermatitis: a comparison with experimental allergic contact dermatitis. J Am Acad Dermatol. 1987; 16: 1175-1179.
Comments are closed.